Casey Derella
PhD Candidate
Augusta University
Microdialysis is a clinical and laboratory research tool used in a variety of fields from drug development, cancer research, physiology, and even ecology. Microdialysis provides insights to biochemical changes in the tissues before systemic blood level detection is possible by monitoring the extracellular space of living tissues. Designed to mimic a blood vessel, a microdialysis probe is a catheter with a semipermeable membrane in the middle or at the end. The semipermeable membrane is placed in the extracellular space and allows for relatively free transport of some but not all solutes by passive diffusion. Permeability, however, is relatively limited to small molecules of less than 20,000 Da in molecular mass. Once in place, fluids, of a known substrate or concentration, are pumped through the probe at a controlled rate (Figure 1), allowing various molecules of interest to be delivered (to test receptor function) or dialysates can be collected for subsequent analyte quantification. Based on the location of the microdialysis probe, collected dialysates represent local tissue concentrations and therefore, allow continuous monitoring of drug delivery and change in local metabolic parameters. With this technique, a number of tissues can be studied and analyzed including adipose tissue, blood, brain, heart, skin, and even the kidney.
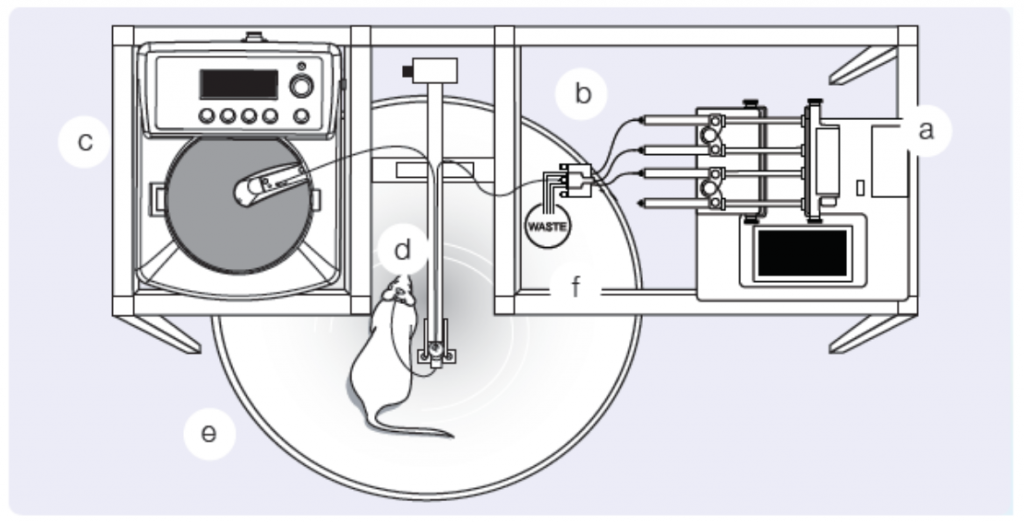
Perhaps more importantly, microdialysis is not only a pre-clinical tool, but can be employed in humans for translational studies. Human cutaneous circulation has been demonstrated to be a valuable, minimally invasive model of generalized microvascular function. Several pathologies, including hypertension, diabetes, and renal disease manifest in microvascular dysfunction. Intradermal microdialysis allows the investigation of the relationship of cutaneous microcirculation with cardiovascular disease and with microdialysis, researchers are able to manipulate the putative signaling pathways associated with vascular pathology and test integrative cutaneous vascular function. In fact, a recent study determined the association between skin microvascular function and renal hemodynamics in type 2 diabetes. This study and many others demonstrate the versatility and salient use of microdialysis in translational human studies of disease. However, our focus today is the use of microdialysis in pre-clinical models to investigate kidney injury and disease.
Microdialysis probes are designed to facilitate interstitial fluid sampling and have been employed in cortical and medullary renal research. Early studies of angiotensin II (Ang II) applied microdialysis and observed higher renal interstitial fluid levels of Ang II compared to plasma levels in dogs and rats. A follow-up study, expanded these previous findings and reported greater levels of Ang II in the renal cortex interstitial fluid compared to arterial blood and kidney tissue levels. These studies were primarily interested in the interstitial fluid levels of the kidney, therefore, a physiological Ringer’s solution was perfused through the microdialysis fibers and collection tubes were utilized to collect the dialysate. The collected dialysate represents the interstitial fluid levels of the local area.
This concept of interstitial fluid sampling has been used to evaluate and detect kidney ischemia by Keller and colleagues in porcine models. Microdialysis fibers were placed superficially in the lateral kidney cortex and the lateral side of the capsule of native kidneys in 20 female Danish Landrace pigs, and the contralateral kidney was removed. Following baseline dialysate collections, 8 of the pigs underwent arterial ischemia, 8 others underwent venous ischemia conditions while the remaining 4 served as time controls. Dialysate was collected every 30 minutes and glucose, glutamate, glycerol, and lactate (measures of anaerobic respiration) were measured on a CMA microdialysis analyzer. As oxygen supply decreases under ischemic conditions there is a switch to anaerobic metabolism, and this was reflected by a significant increase in cortical glutamate, glycerol, and lactate 30 minutes after arterial or venous ischemia. However, glucose decreased after 60 minutes in the venous ischemia group compared to 90 minutes after arterial ischemia. Glutamate was the only substrate to increase in and outside of the kidney in the ischemic pigs. To put this in perspective, in healthy conditions, glutamate is reabsorbed in the proximal tubules, but this transportation is inhibited by high extracellular concentrations of potassium during ischemia. This observation suggests that glutamate may be the most reliable substance for early detection of renal ischemia. Overall, the microdialysis fibers placed in and just outside the kidney were able to detect metabolic changes induced as a result of renal ischemia within 30 minutes.
Renal microdialysis is a unique and versatile tool to monitor local metabolic changes in the non-human kidney. It has been demonstrated in a number of preclinical studies to accurately measure various metabolites in the local interstitial fluid. On the other hand, microdialysis can also be used in human studies to monitor interstitial fluid or test the function of microvascular beds. For example, a 2014 study utilized the cutaneous circulation to test the hypothesis that reactive oxygen species (ROS) derived from NADPH oxidase and xanthine oxidase impair nitric oxide-dependent vasodilation in people in stage 3-4 chronic kidney disease (CKD). Ringers solution (control site), tempol (ROS-scavenger), apocynin (NADPH oxidase inhibition), and allopurinol (xanthine oxidase inhibitor) were perfused through microdialysis fibers place in the intradermal layer of the forearm of patients with CKD. Overall, the cutaneous skin blood flow was reduced in people with CKD but augmented with tempol and apocynin, suggesting that NADPH oxidase is a large source of oxidative stress in people with CKD. This excess production of oxidative stress by NADPH oxidase is likely contributing to microvascular dysfunction. Interestingly, the literature supports the use of this technique to correlate with other vascular beds, such as the renal microcirculation. Overall, microdialysis demonstrates to be a multifunctional research tool for basic and clinical scientists.
Reviewed by: Elinor Mannon, Kelly Hyndman, PhD, Matthew Sparks, MD, FASN, FAHA

i like this helpful article, thanks for sharing this info