 What are the most common type of cancers in kidney transplant recipients?
What are the most common type of cancers in kidney transplant recipients?
The three most common cancers are: non-melanoma skin cancers, Kaposi’s sarcoma and non-Hodgkin lymphomas. Compared to the general population, the risk of squamous cell carcinoma is 100x greater in transplant recipients. One interesting aspect about transplant recipients is that they have a much higher risk for a few specific malignancies but are not broadly predisposed to all cancers. As an example, the incidence of most common solid tumors in the general population (lung, prostate, breasts and colorectal) is only modestly increased or similar to the transplanted population. This suggests that other factors might play a role in the pathogenesis of malignancy post-transplantation.
What is the cause of increased risk of malignancies after transplantation?
There are several factors that seem to be associated with the development of malignancy after transplantation: impaired immune surveillance secondary to immunosuppression; carcinogenic factors like sun exposure; and host factors such as genetic predisposition to cancer, presence of oncogenic viral infection and prolonged dialysis.
How does a viral infection may increase the risk of malignancy?
Certain virus like EBV, HHV-8 and HPV carry viral oncogenes in their genome, which directly affect cell-cycle and apoptosis regulation. For example, HPV has the E6 oncogene that produces a protein that binds to p53, a major regulator of cell growth and tumor suppressor protein. Following E6 binding of p53, p53 is degraded, allowing unchecked cellular cycling and accumulation of chromosomal mutations without DNA repair.
Do different immunosuppressive drugs vary in their risk of post-transplant malignancy?
In general, the intensity and duration of immunosuppression therapy plays a major role in determining this risk of malignancy, since it severely disrupts the immune surveillance of cancers cells and dampen anti-viral immunity. Moreover, certain specific drugs like azathioprine and cyclosporine have been shown to directly lower the repair rate of DNA damage induced by solar UV radiation. In contrary, mTOR inhibitors have been shown to inhibit tumor growth mediated by both blockade of the PI3K-Akt-mTOR pathway, which is frequently activated in cancer, and by angiogenesis inhibition.
Compared to the general population, are there special screenings required for the kidney transplant recipient?
Since skin cancer is one of the most prevalent malignancies, recipients should be educated about their increased risk and recommended to reduce levels of sun exposure (especially between 10am and 4pm), wear protective clothing and apply sunscreen to sun-exposed areas, perform self-examinations and annual skin examinations by qualified health professional. For non-skin cancers, the KDIGO guidelines recommend solely reinforcement of the regular cancer screenings performed in the general population.
If a malignancy arise, what is the ideal approach?
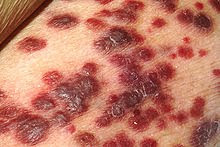
The cancer should be managed with specific therapies for the particular tumor type associated with reduction and/or conversion to a mTOR inhibitor. The conversion to mTOR inhibitors have been successful in inducing complete regression of Kaposi’s sarcoma (figure) and decreasing the recurrence of skin cancers. General contraindications for conversion in kidney transplant recipients are proteinuria greater than 500mg/day and GFR less than 40 ml/min.
Gap in knowledge: we still need some prospective trials in order to determine if further cancer screening exams would be warranted in the transplant population that carries other competing risks like cardiovascular disease and infection.

Ah if only there wasn't as many side effects with sirolimus.
My husband recently contracted Hodgkins lymphoma (last year). He went into remission very quickly and is doing well now but he's had to reduce his cyclosporin dosage and will likely be increasing his Cellcept dosage. Sirolimus was also offered but Cellcept is known to work for him so we are opting for this change.
As of last month, he has had his transplanted kidney for 20 years. Let's hope that his transplanted kidney continues to work without futher malignancies in future for as long as possible.
Thank you to all the specialists who update this blog.
Whilst I am not (yet) working the medical field (have started studying in the field though), this blog is really a well of knowledge.
Great question. The major limitation for sirolimus use in transplantation is its side effect profile (renal toxicity and bone marrow suppresssion) and its low immununosuppressive potency. The long-waited ELITE-Symphony Study revealed that the incidence of acute rejections was higher in patients administered rapamycin than in those administered either tacrolimus or ciclosporin. Nonetheless, as we learn to better use this agent, it might still have some beneficial effects in combination with other drugs.
So, why not mTOR as first-line for most?, (hence perhaps preventing more of these cancers).