Introduction
Ideally, patients with end-stage kidney disease would begin hemodialysis with a mature, functional arteriovenous access. However, in certain subgroups of patients, central venous catheters (CVCs) are unavoidable. Even for patients who ultimately use an arteriovenous fistula (AVF) or arteriovenous graft (AVG) for long-term dialysis, the significant majority are exposed to CVCs and their associated risks at some point in their lives.
CVC complications may be broadly categorized as infectious or non-infectious. Infectious complications include catheter-related bloodstream infection (CRBSI) and tunnel or exit site infections. Non-infectious complications include mechanical issues (e.g., malpositioned or kinked catheter), central vein stenosis, or thrombosis. In this post, we will explore some common CVC-related complications and how to manage them.
Infectious Complications
Case 1: You are notified by the dialysis nurse that your patient didn’t quite seem like her normal self when she came to dialysis today. Halfway through her session, she complained of nausea and chills. Oral temperature is 101 F. She dialyzes through a CVC and you immediately suspect CRBSI. Your next step is to:
a. Immediately start treatment with empiric broad-spectrum antibiotics
b. Order a STAT chest x-ray and urinalysis
c. Ask for blood cultures to be drawn from the peripheral veins
d. Ask for blood cultures to be drawn from the catheter lumen and the dialysis circuit
Case 1 offers an example of CRBSI. The risk for developing CRBSI increases the longer the catheter is used. Patients commonly present with fever and chills, but occasionally have other non-specific symptoms such as malaise or encephalopathy. Obtaining peripheral blood cultures in dialysis patients is often challenging, and blood cultures drawn from the catheter hub and the dialysis circuit are most accurate for diagnosing CRBSI in the hemodialysis population (choice d). Patients should be treated with empiric broad-spectrum antibiotics while awaiting culture results. Type and duration of antibiotic therapy should be tailored based on antibiotic sensitivities. Catheter management may vary depending upon the patient’s response to antibiotics and the infecting organism (Figure 1 below).
Case 2: You are asked to evaluate a CVC-dependent dialysis patient who presented to the Emergency Department. He reports subjective fevers over the last 3 days and notes that the area around his right internal jugular CVC is red and sore. You obtain blood cultures and cultures of drainage at the exit site and start empiric antibiotics. Cultures come back positive for methicillin-resistant Staphylococcus aureus. In addition to giving appropriate antibiotics with dialysis, you plan to:
a. Remove the current catheter and place a new catheter at a different location
b. Lock the catheter with an antibiotic solution following dialysis
c. Order washout of the tunnel and guidewire catheter exchanged
d. Treat the exit site infection with topical mupirocin
Case 2 represents an exit site infection with concurrent bacteremia. It is seen almost exclusively with Staphylococcal infections. Catheter salvage is reasonable with Staphylococcus epidermidis, but if the infecting agent is Staphylococcus aureus, the catheter should promptly be removed and a new catheter placed in a different location once bacteremia has cleared (choice a).
Non-Infectious Complications
Case 3: You are rounding in the dialysis unit when the nurse approaches you about a CVC-dependent patient whose machine keeps alarming for high arterial pressures. This has happened before and has usually improved with thrombolytics, but now he can only achieve a maximum blood flow rate of 200 mL/min despite a 1-hour alteplase dwell prior to dialysis. You decide to:
a. Ask the nurse to reposition the patient and try to reverse the dialysis ports
b. Refer the patient for CVC exchange
c. Order a three-hour alteplase dwell
d. Order ultrasound vein mapping and surgical consultation for AVF placement
Case 3 suggests the presence of a fibrin sheath, which can begin to form as soon as 24 hours following placement of the catheter. A fibrin sheath is composed of fibrinogen, lipoproteins, albumin, and coagulation factors. It has been found in up to 70% of catheters being exchanged for malfunction. The best choice for this patient, who has failed to improve following tPA dwell, is referral for CVC exchange with possible angioplasty and/or stripping of the fibrin sheath (choice b).
Case 4: You have a patient with a history of two failed right upper extremity AVFs and who is currently dialyzing through a left subclavian vein CVC. She had a new AVF placed in the left upper arm 6 weeks ago. Based on a recent ultrasound, the AVF is mature, but it is too deep and needs to be superficialized before it can be used. She has been complaining of left arm pain and swelling during her last few dialysis sessions. You are concerned for:
a. Left upper extremity AVF stenosis
b. Left upper extremity deep vein thrombosis
c. Central vein stenosis
d. Right upper extremity deep vein thrombosis
Case 4 is consistent with central vein stenosis (choice c). Subclavian catheters are associated with a higher risk of developing central vein stenosis compared with internal jugular catheters, as are left-sided catheters, a history of peripherally inserted central catheters (PICCs), recurrent catheter placements, and longer catheter use. Symptoms tend to arise when there is a functional AV access on the same side as the catheter.
Angiography is the preferred method for diagnosis. Patients with central vein stenosis may be asymptomatic and do not always require catheter removal unless problems develop. Angioplasty (with or without stenting) is the treatment of choice, followed by surgery for recurrent vein stenosis where angioplasty has failed.
Prevention of Thrombotic Complications
Heparin or citrate solution may be used to lock the catheter between dialysis sessions to help prevent catheter-related thrombosis. Routine prophylactic use of systemic antiplatelet agents or warfarin has not been proven to help prevent thrombotic complications, and may increase the patient’s bleeding risk. Avoiding catheters altogether or removing catheters as appropriate is the best way to prevent catheter-related thrombosis.
Summary
General bedside management for non-infectious catheter complications includes repositioning the patient, forceful flushing of saline through the catheter, tPA dwell prior to dialysis, or obtaining a chest x-ray to rule out catheter kinks or malpositioning. If conservative measures fail to improve catheter function, the patient should quickly be referred to an interventional radiologist or nephrologist for further evaluation.
| Finding | PossibleSigns/Symptoms | What To Do If Suspected |
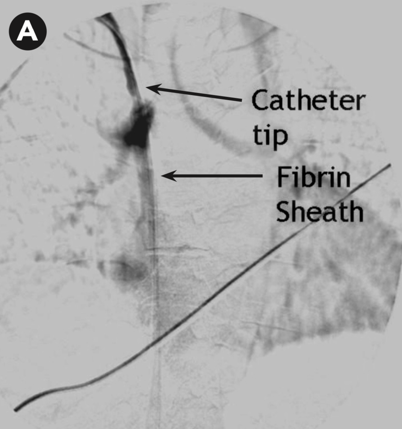 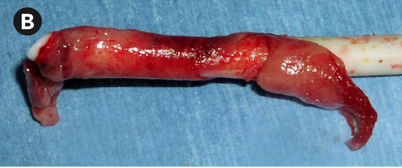 Fibrin sheath. Vachharajani, Atlas of Dialysis Vascular Access, 2010. | Difficulty aspirating from the catheter High arterial and/or venous pressures on dialysis Poor blood flows Laboratory evidence of decreased clearance (URR < 65% or Kt/V < 1.2) | Reposition the patient Obtain a chest x-ray to check for catheter kinks Forceful saline flush Attempt thrombolytics (tPA 1 mg per lumen) If thrombolytics are ineffective, consult interventional radiology or nephrology for catheter exchange with possible angioplasty/stripping of the fibrin sheath |
 Kinked dialysis catheter. Vachharajani, Atlas of Dialysis Vascular Access, 2010. | Difficulty aspirating and flushing the catheter Poor blood flows | Obtain a chest x-ray Release tight sutures Consult interventional radiology or nephrology for catheter reposition or guidewire catheter exchange |
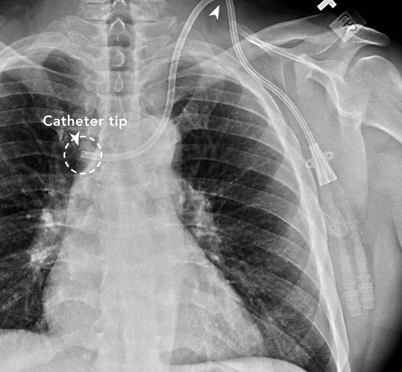 Malpositioned dialysis catheter. Vachharajani, Atlas of Dialysis Vascular Access, 2010. | Difficulty aspirating from the catheter High arterial or venous pressures on dialysis Poor blood flows | Obtain a chest x-ray to check the location of the catheter Consult interventional radiology or nephrology for guidewire catheter exchange |
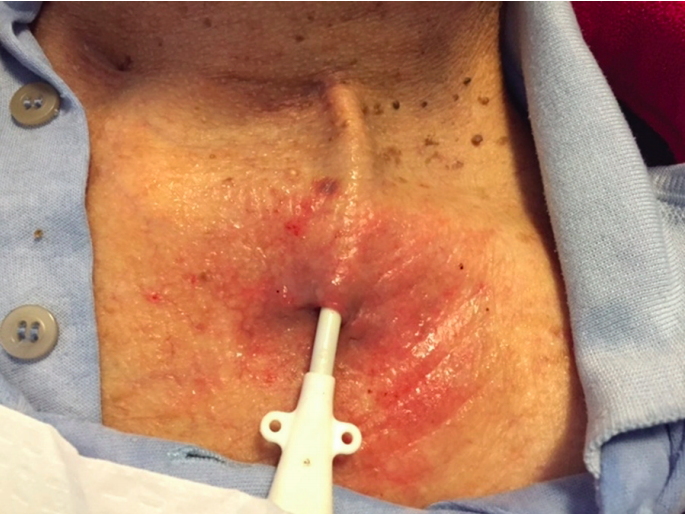
 Exposed cuff. Vachharajani, Atlas of Dialysis Vascular Access, 2010. | Visible cuff outside of the exit site | Do not use catheter for dialysis Consult interventional radiology or nephrology for guidewire catheter exchange |
 Central vein stenosis. Vachharajani, Atlas of Dialysis Vascular Access, 2010. | Ipsilateral arm swelling, breast swelling, facial swelling Dilated collateral veins in the chest, shoulder, or upper arm Possible superior vena cava syndrome | Consult interventional radiology or nephrology for angiogram with possible angioplasty and catheter exchange Removal of catheter if appropriate (i.e., patient has a functional AV access) |
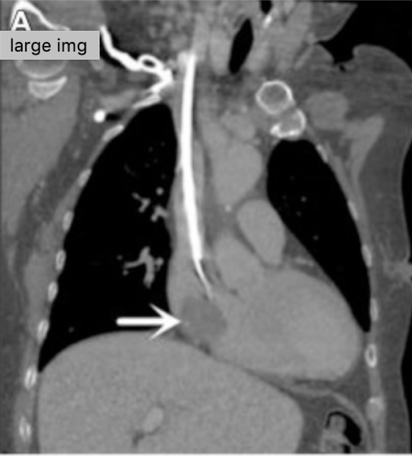 Catheter-related atrial thrombus. Yang et al., JVS, 2018. | Frequently asymptomatic, incidental finding on imaging (95-99% of cases) | Systemic anticoagulation with warfarin or novel anticoagulant as for treatment of DVT Catheter removal or exchange (Figure 2) |
Table 1. Non-infectious hemodialysis contraindications and their suggested management.
Post by:
Crystal Farrington, ASDIN Fellow
Acknowledgements: This post is part of a collaboration between the Renal Fellow Network and the American Society of Diagnostic and Interventional Nephrology (ASDIN), whose mission is to provide excellence in dialysis access care to improve outcomes for patients with kidney disease. Special thanks to Michael Allon, Tushar Vachharajani, Aisha Shaikh, Edgar Lerma, and the ASDIN Education Committee. For more information about the ASDIN mission or membership, click here.



1 comment