Hyponatremia from the Syndrome of Inappropriate Anti-Diuretic Hormone (SIADH) is a common consult a fellow encounters on the inpatient service. In addition to stopping potentially offending drugs, management can range from fluid restriction, loop diuretics, demeclocycline, salt tablets, or urea. Utilization of these options may be met with varying success. More recently, vasopressin receptor antagonists have been added armamentarium because as they target the pathophysiology of SIADH. The recommended starting dose of tolvaptan is 15 mg. However, a recent case series of patients with heart failure and SIADH showed variable rates of Na correction and suggested to start tolvaptan at 7.5 mg to avoid an overaggressive water diuresis that may result in too rapid a correction in the serum sodium. We present two fictitious cases designed for educational purposes to illustrate this point.
Patient 1
A 70-year-old woman is seen emergency department due to new onset of confusion and falls. Her workup resulted in a diagnosis of diffuse large B cell lymphoma. During her admission, her serum sodium (SNa) ranged between 130-141 meq/l and she was eventually transferred to the rehabilitation unit. Over the next few days, her SNa decreased to 127 meq/l and then to 124 meq/l with urea nitrogen of 13 mg/dL. She appeared euvolemic and her mental status was unchanged. Her urine osmolality (UOsm) was 450 mosm/kg and urine sodium (UNa) 130 meq/l, both consistent with SIADH in the setting of her CNS malignancy. Thyroid and adrenal testing were normal.
The patient was following a 1L daily fluid restriction. She was initially given 20 mg of IV furosemide with subsequent 250 ml of urine output, though her repeat SNa dropped to 125 meq/l, and SNa was 123 meq/l by the next morning. The decision was made to give her 7.5 mg of tolvaptan, with subsequent urine output (UOP) of 150 ml and a SNa of 124 meq/l (Figure 1). She had a similar poor response to 15 mg and later the dose was increased. With 30 mg tolvaptan daily, her SNa plateaued around 130 meq/l.
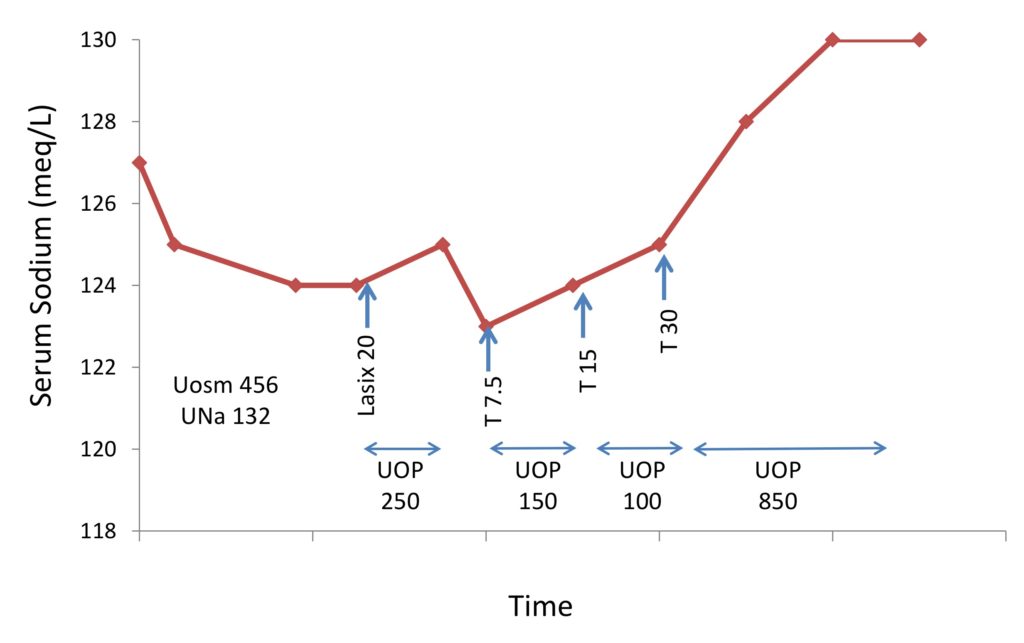
The course of patient 1 and response to 7.5, 15 and 30 mg of tolvaptan (T). UNa = urine sodium in meq/l. Uosm = urinary osmolality in mosm/kg. UOP = urine output in ml.
Patient 2
A 90-year-old woman presents to the ED with confusion and cough. Her workup was consistent with pneumonia. Initial labs showed a SNa of 120 meq/l, urea nitrogen 9 mg/dL, Uosm 780 mosm/kg, and UNa 65 meq/l, consistent with SIADH in setting of an acute lung process. She appeared euvolemic and her thyroid and adrenal testing were normal.
As management with hypertonic saline was not in line with goals of care, she received 7.5 mg tolvaptan and over the next 8 hours she produced 2500 ml of urine. Her SNa increased to 128 meq/l. She produced another 1200 ml UOP over the next shift and her SNa continued to rise to 133 meq/l. Over the course of the first 30 hours, she made a total of 5.3 L and required 5% dextrose in water to limit the change in sodium to 11 meq/L. (Figure 2).
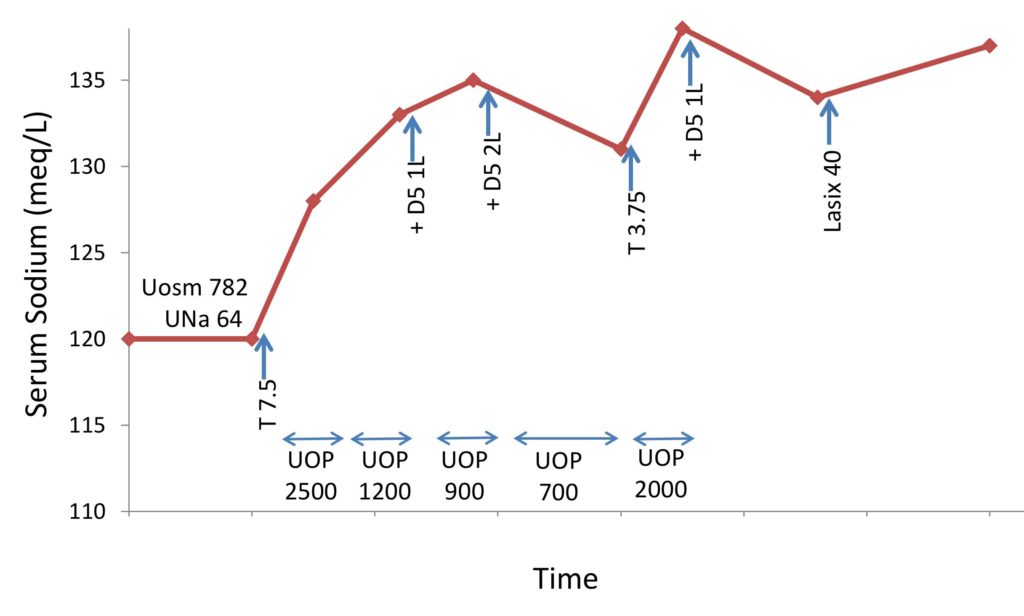
The course of patient 2 and response to 7.5 and 3.75 mg of tolvaptan (T). UNa = urine sodium in meq/l. Uosm = urinary osmolality in mosm/kg. UOP = urine output in ml.
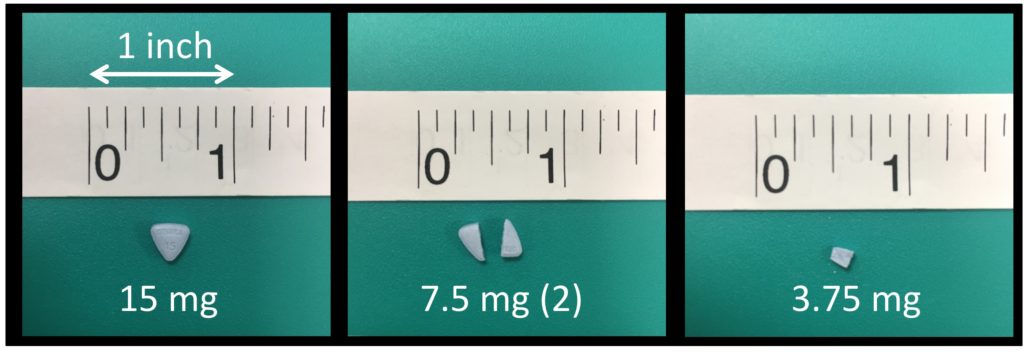
The next day, she received a 3.75 mg dose of tolvaptan, requiring dividing of a triangular pill into quarters (Figure 3). This also had a robust effect, leading to another 2L of urine output and an increase in SNa from 131 meq/l to 138 meq/l within 6 hours. Again, sodium had to be lowered with hypotonic fluid infusions. Tolvaptan was then discontinued and further management was pursued with loop diuretics
Thus, as you can see both patients responded much differently to tolvaptan administration. In the initial SALT trials, the starting dose was 15 mg but could be increased up to 60 mg. In the SIADH subset, only 5.9% (3/51) of patients exceeded the maximum correction rate (>12 meq/l over the first 24 hours). In Europe, post marketing assessments for tolvaptan found that physicians were splitting tablets to allow for a 7.5 mg starting dose out of concern for over correction. A subsequent manufacturer sponsored pharmacodynamic study found that the 15 mg, 7.5 mg, and 3.75 mg doses were all susceptible to over correction (>8 meq/l in 8 hours).
Interestingly, both the 7.5 mg and 3.75 mg doses resulted in similar free water clearance and urine volume. In Italy, a separate study published in 2016 found a 42% over correction rate (>12 meq/l in 24 hours) with the 15 mg dose compared to none in the 7.5 mg group. Recently, Morris et al. examined the use of daily tolvaptan of 15 mg in 28 patients with SIADH and 39 patients with CHF. In patients with a SNa less or equal to 130- 33% of patients with SIADH over corrected (> 8 meq over 24 hours) compared to 5% of the CHF group. Lower baseline SNa (<121 meq/l) and lower baseline serum urea nitrogen (<10 mg/dL) were associated with more rapid 24-hour correction rates. These data show need for caution when using tolvaptan in patients with SIADH and to consider starting the dose less than 15 mg as overcorrection can lead to osmotic demyelination syndrome (ODS). While the reporting of ODS with tolaptan use have been rare most have been with the concomitant use of hypertonic saline or diuretics. However, it is imperative to remain hyper vigilant about overcorrection as ODS development can lead to permanent neurological damage.
Tovaptan may be an attractive option for SIADH as it directly targets pathophysiology, and let’s face it, as nephrologists we can appreciate dilute urine. However, it is expensive with the risk of potentially serious adverse effects.
In A Tale of Two Cities Dickens wrote: “It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness…”; we find this quote fitting for our experience with these two patients.
Post by: Milani Sivagnanam, MD
Chief Nephrology Fellow, Rush University Medical Center



Having the same experience also with this 15 mg where the patient’s sodium level was corrected like crazy and her urine out put made me dizzy 🙁
This is very interesting and highlights why I do not use this drug clinically. I have never prescribed a vaptan for a patient – I came close last year in a patient with intractable hyponatremia and heart failure – and I don’t see a situation where I would use it for SIADH. They are expensive with unpredictable effects and a very high risk for overcorrection as seen in the second case in particular.