When consulted by your infectious disease colleagues as to the cause of new or worsening renal impairment in a patient with human immunodeficiency virus (HIV), the differential is broad but changing. The classic HIV-associated collapsing glomerulopathy has become less common in the era of combined antiretroviral therapy (cART) and the prevalence of other kidney disease in persons with HIV has increased. The issue is excellently summarized in this KDIGO executive conclusions [2018] article on kidney disease and HIV. Severe kidney impairment secondary to cART alone is rare, but nephrotoxicity does occur and one of the challenges in treating these patients is keeping up to date with the expanding repertoire of drugs used in their care.
In this post, we will look at the common nephrotoxicities seen in association with cART. Antiretroviral (ARV) drug names (and their constituents) are summarized in this chart.
1. Tubular Injury
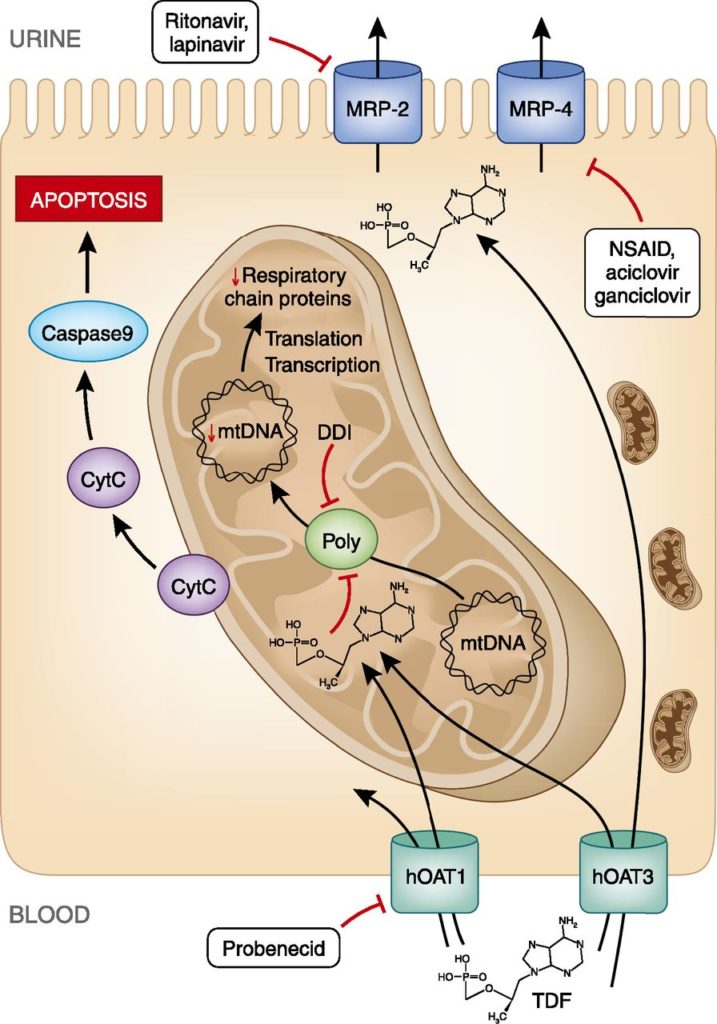
The archetype of ART-associated nephrotoxicity is tenofovir disoproxil Fumarate (TDF) proximal tubular injury presenting with Fanconi Syndrome.
In reality, TDF tubulopathy can present insidiously with preserved GFR, low-grade proteinuria and phosphaturia. Progression to severe, treatment-limiting tubulopathy, characterized by osteomalacia and fragility fractures, metabolic acidosis, normoglycaemic glucosuria and progressive decline in GFR, occurs in about 1% to 2% of patients receiving this medication. The precise mechanism of TDF tubulopathy has yet to be fully elucidated but postulated mechanisms are summarised by Fernandez et al in their 2011 review.
Hamzah et al studied risk factors for developing TDF tubulopathy and report increased incidence with concomitant use of didanosine and protease inhibitors, duration of treatment, older age, Caucasian race, and immunodeficiency.
There is some evidence that switching TDF to the prodrug Tenofovir Alfenamide (TAF) is associated with improvements in renal function. One study found that a switch from TDF to TAF compounded with elvitegravir, cobicistat and emtricitabine was associated with improvements in proteinuria and bone mineral density at 96 week follow-up without a further decline in GFR.
2. Crystalluria and Nephrolithiasis
Crystalluria and nephrolithiasis are seen in association with the protease inhibitors (PIs) darunavir, indinavir and atazanavir. Indinavir is the classic MCQ vignette drug for crytalluria in the setting of a person with HIV on cART but is no longer considered a first-line PI. Although the majority of patients with indinavir-associated cystalluria will be asymptomatic, there are reports of patients on indinavir developing AKI secondary to obstructive uropathy requiring urological intervention. In a single-centre study, the newer and more widely used PI Atazanavir, when boosted with ritonavir, was associated with increased risk of nephrolithiasis.
3. Inhibition of the Tubular Secretion of Creatinine
The renal effects of novel antiretroviral drugs (particularly, integrase inhibitors) were summarised in a review article by Milburn et al (2016). Experimental data suggests that the integrase inhibitor Dolutegravir inhibits tubular secretion of serum creatinine by the basolateral organic cation transporters without a true reduction in GFR. The same may be true of Raltegravir on the basis of data from the SAILING and SPRING-2 studies. There is, however, a smaller study that showed a reduction in GFR measured by cystatin-C clearance in patients who switched from efavirenz to raltegravir. There are no reports that Elvitegravir is associated with any renal adverse effect. Finally, the drug Cobicstat or COBI, which has no ARV activity and is used as a pharmaco-enhancer, increases serum creatinine levels by inhibiting tubular secretion of creatinine by the apical membrane transporter MATE1.
4. Lactic Acidosis
NRTI-associated hyperlactataemia is well described in the literature. The presentation ranges from a benign, asymptomatic elevation in serum lactate (2.1 – 5mmol/L) to a severe lactic acidosis syndrome with high mortality (>50%). The NRTI class medications most frequently implicated are Stavudine and Didanosine.
Conclusions
The kidneys can be affected by ARV medications. Consideration of drug-related nephrotoxicity should be a part of the comprehensive assessment of renal dysfunction in patients with HIV. Particular care should be taken when assessing patients taking the integrase inhibitors dolutegravir and (perhaps) raltegravir, both of which may cause an elevated serum creatinine without true loss of GFR.
Post by: Ted FitzGerald, MB MRCPI
NSMC Intern, 2019
Nephrology Specialist Registrar
University Hospital Waterford, Ireland