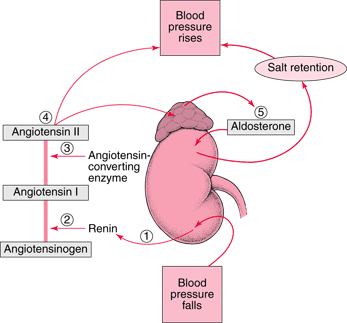
 Researchers from the UK published a new and exciting article exploring one of the fundamental mechanisms of blood pressure regulation by the renin-angiotensin-system (RAS). This research was, per the authors, 20 years in the making and was fittingly published this week in Nature. The RAS is a multi-enzymatic system with multiple layers of control (click here for a review of the RAS from Nate). The first step of this process is the cleavage of angiotensinogen by the enzyme renin to form angiotensin I (Ang I). Precisely how renin acts upon angiotensinogen has not, until now, been completely elucidated.
Researchers from the UK published a new and exciting article exploring one of the fundamental mechanisms of blood pressure regulation by the renin-angiotensin-system (RAS). This research was, per the authors, 20 years in the making and was fittingly published this week in Nature. The RAS is a multi-enzymatic system with multiple layers of control (click here for a review of the RAS from Nate). The first step of this process is the cleavage of angiotensinogen by the enzyme renin to form angiotensin I (Ang I). Precisely how renin acts upon angiotensinogen has not, until now, been completely elucidated.
The researchers were able to almost completely resolve the structure of angiotensinogen (a large 452 AA protein) by x-ray crystallography to the resolution of 2.1A. This showed that the angiotensin cleavage site was inaccessibly buried in the c-terminal tail of this large protein. They went on to show that when angiotensinogen is oxidized this region changes shape to permit ready access of this site to renin which, in turn, cleaves off a 10 amino acid portion termed Ang I. The supplemental data shows a nice 3D movie of this interaction. The oxidized form of angiotensingen is able to bind to the pro-renin receptor in tissues and has a 4-fold higher catalytic activity for Ang I formation than the reduced form. They authors hypothesize that under conditions of oxidative stress, angiotensinogen is found more often in the oxidized form leading to hypertension.
Lastly, as proof of principle, they showed that patients with preeclampsia had a higher amount of the oxidized form of angioensinogen in their plasma. Typically the ratio of reduced to oxidized angiotensinogen is 40:60 in normal individuals. In preeclampia this ratio is 30:70. They speculate that oxidative stress may be sufficient to cause hypertension associated with preeclampsia. Interestingly, a mutation in the angiotensinogen gene has been reported to cause increased catalytic activity this protein.
In conclusion, this report opens up many new investigative avenues in the field of hypertension research. Not only does this report hint at a possible role for anti-oxidants in hypertension therapy, but a new target for drug modulation of this complex system now exists. However, more research will be needed to confirm how changes in angiotensingen structure leads to alterations in blood pressure homeostasis.

