folktale: an old and very senile king wants to know which of his daughters
loves him the most. The elder sister says she cherishes him more than the whole
kingdom. The younger one, though, loves him “as dear as meat loves salt”. The
king gets upset and orders her execution. As most fairy tales,
it has a happy ending: the king is
served dinner without salt, learns his lesson, stops the execution and everyone lives happily ever
after. In the modern world this story of a salt-loving family might have
another sad “end” – end stage kidney disease, and a broke king spending a lot
of gold coins on healthcare. It might have ended differently for him though if
we had a better understanding of renal electrolyte handing during a high salt
diet. This is a million-dollar question (actually, it’s more like
hundreds-of-millions-of-dollars-question – I hope someone at NIH reads this blog); we need to
know what happens in the kidney at the molecular and cellular level in a disease
state. Knowing all the tiny details would be immensely helpful to discover new pharmacological targets.
One of the most studied drug targets for renal diseases are ion channels, and electrophysiology is a sophisticated method for measuring ion channel activity.
In my lab at the Medical
University of South Carolina among other things we use electrophysiology to uncover
the mechanisms that underlie salt-sensitive hypertension. Since the Nobel Prize in Physiology and
Medicine awarded to Erwin Neher and Bert Sakmann for their “discoveries
concerning the function of single ion channels in cells” in 1991,
electrophysiology has been an immense help to basic and clinical science. Although
this technique is very widespread in brain and cardiovascular research, there
is only a handful of labs that use it to answer nephrological questions. I have
learnt it during my postdoctoral training in the laboratory of Dr. Alexander
Staruschenko (Medical College of Wisconsin) where it has been successfully applied
to study kidney function for years.
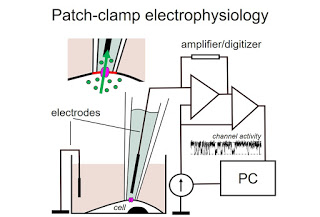
how ions (Na+, K+, Cl-, Ca2+ – you
name it) move through ion channels in the cell membrane. Using a tiny glass
micropipette with an electrode in it, and a micromanipulator, we can press the
tip of the pipette to plasma membrane of the cell, and isolate a patch of it
that is under the tip (hence the other name of the approach – patch clamp). Since ions have a charge,
their movement through the channel to the other side of the membrane generates
current that can be sensed by the microelectrode in the pipette. Of course,
sensing movement of single ions requires sophisticated amplifiers, filters, digitizers,
grounding, knowing Ohm’s law and having a graduate degree in physics (kidding –
though I have one, and it does help), and a ton of patience. When performing a
patch-clamp experiment we control the environment that the cell is in (we
choose the extracellular solution, what we put in the patch micropipette, what
voltage we apply to the membrane etc), and this allows us to use different
tricks to identify ion channels and then learn how to tweak or test their
activity. Using cultured cells, tissues isolated from animal models, or human
biopsies, we can directly measure the activity of ion channels and assess what
happens in a certain disease condition.
Over the years,
scientists have discovered dozens of crucial renal ion channels conducting sodium,
calcium, chloride, potassium, magnesium… more are discovered every year. Gain-
or loss-of-function mutations that lead to renal diseases have been reported
for pretty much each and every one of them. In disease states such as
salt-sensitive hypertension a handful of ion channels are known to work
improperly, and in order to advance our treatment strategies we need to know
how these channels are mediated, what signaling pathways affect them, and what
pharmacology can be used to change their activity. As an example, in Dr.
Staruschenko’s laboratory it has been shown that epithelial sodium channel
(ENaC) in the cortical collecting ducts can be overly active in salt-sensitive hypertension
(PMID: 28003189), which meaningfully contributes to an increase in blood
pressure. Therefore, specific targeting this ion channel could be a successful
means to combat blood pressure in this setting.
colleagues once compared ion channel electrophysiology to fishing. I have never
fished in my life, but since I’ve done a lot of patch-clamp I feel I could easily
have an alternative career as a fisherwoman. Nevertheless, although patch clamp
electrophysiology is a “long wait – high reward” approach, this unique
technique is crucial for our understanding of renal electrolyte handling, and –
in skilled and patient hands – it has tremendous potential to push the medical field
forward.
PhD,
Medical University of South Carolina
Nephrology