As a medical student, I encountered two cases of pulmonary renal syndrome within a few months of each other. In both cases, both anti-neutrophil cytoplasmic antibody (ANCA) and anti-glomerular basement membrane (GBM) antibodies were identified (the so called “double positive” disease). Double positive disease can be defined as an ANCA vasculitis (AAV) phenotype on biopsy plus anti-GBM antibody presence or anti-GBM phenotype on biopsy plus ANCA positivity (Figure 1).
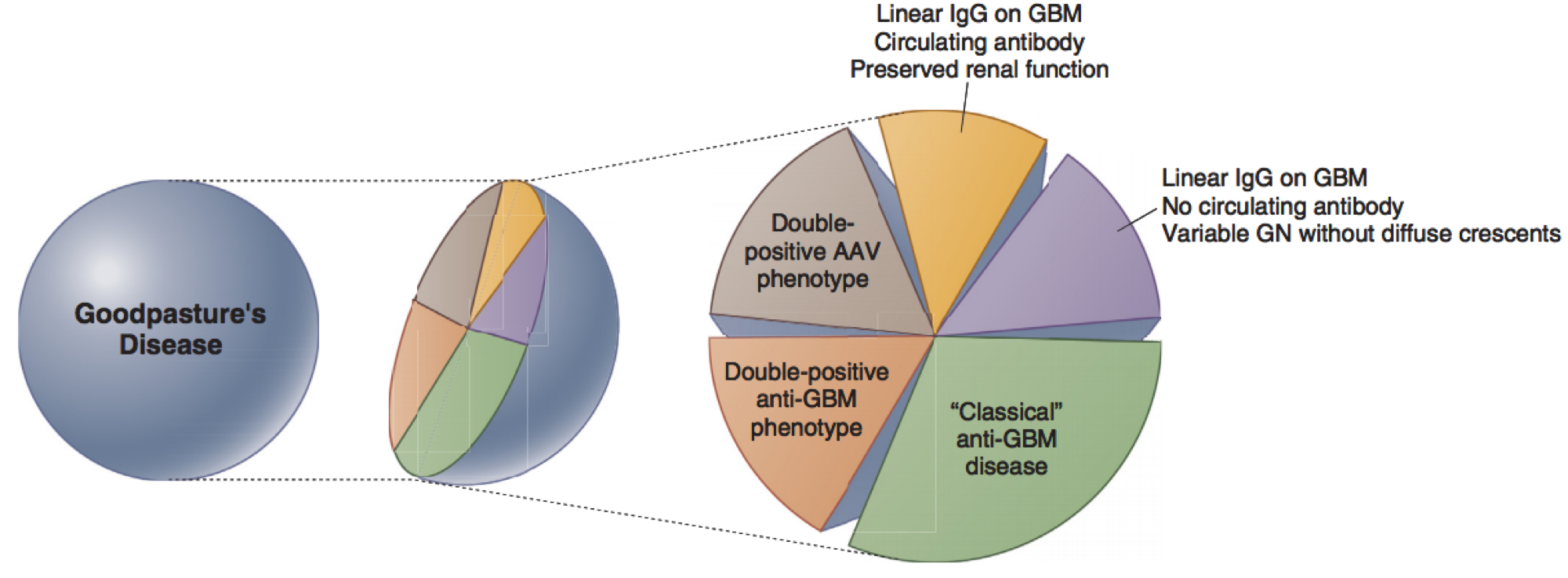
How often double positivity occurs: Acute GN due to anti-GBM disease is rare, occurring in less than one case per million. The incidence of ANCA-associated vasculitis (AAV) in the US is 33 per million. Studies have shown that 30-38% of patients with anti-GBM antibodies are also ANCA positive, while 5-14% of patients with ANCA antibodies are anti-GBM positive. While ANCA and anti-GBM antibodies are antigenically distinct and the “cross-over” is not due to cross-reactivity, their association is not fully understood.
Albany Medical Center (AMC), my alma mater, recently published a retrospective study of 41 cases of AAV with kidney involvement over about 12 years, 4 of which were double positive disease (defined as biopsy proven pauci-immune GN and positive anti-GBM antibody). Although referral bias should certainly be considered at a tertiary care center, 37 of the 41 cases were referred locally with 16 patients living within a 15-mile radius of each other – perhaps suggesting a higher regional trend of AAV. High quality population-based studies are lacking to investigate environmental or genetic factors in AAV due to its low incidence. Small observational studies suggest there may be a role for farming/silica exposure.
How double positivity impacts prognosis: Investigating the serologies is not purely academic. It provides important predictive and prognostic factors. Several small studies have been published comparing clinical features and outcomes among patients single positive (SP) AAV, SP anti-GBM, and double positive disease with conflicting results given the rarity of these diseases and subsequently small sample sizes. The largest of these studies was a recent retrospective study including four European centers, which revealed that AAV and patients with double positive disease (defined as biopsy proven anti-GBM disease with dual antibody positivity) tended to present at an older age. Patients with single positive anti-GBM disease were more likely to present in the typical bimodal age distribution. Patients with double positive and single positive anti-GBM had similar severity at disease presentation, with 1/3 developing lung hemorrhage and approximately 60% requiring renal replacement therapy (RRT). Thus, the authors conclude that there is an “anti-GBM phenotype” that dominates early in the presentation of double positive disease.
Though survival was similar in all three groups, overall survival was affected by the severity of disease (age at diagnosis, requirement for RRT, presence of pulmonary hemorrhage on presentation). No patients with single positive anti-GBM disease experienced disease relapse, but half of surviving patients with AAV and double positive disease had recurrent disease during a median follow-up of 4.8 years. The risk of progression to ESKD was intermediate in double positive disease (Figure 2).

The bottom line: Although patients with double positive disease, may manifest like anti-GBM disease on presentation, they were found to share important prognostic features with patients with AAV. Patients with double positive disease had higher rates of renal recovery after dialysis (29%) than patients with single positive anti-GBM (17%). The authors conclude that although patients with double positive disease may reflect single positive anti-GBM disease phenotypically earlier on, like AAV, they require frequent long term follow up and should be considered for maintenance immunosuppression. Thus, knowing both the patient’s ANCA and anti-GBM antibody status in patients with rapidly progressive crescentic GN is important to guide management and predict prognosis.
Post by: Abigail Belasen, MD (@arbelasen)
PGY-1, Department of Medicine
Icahn School of Medicine at Mount Sinai (@DOMSinaiNYC)


