For lovers of an underdog, the story of Tamm Horsfall protein is one of the coolest in science. Discovered in the 1950s, Tamm Horsfall protein was acknowledged but largely ignored for decades, the quirky fun uncle of the urine.
On a physical level, Tamm Horsfall protein is a glycoprotein which is only expressed in the thick ascending limb of the kidney. It’s one of the most abundant proteins in the urine- up to 50mg are excreted a day. A small amount is also released “backward,” through the basolateral part of the cell and into the circulation. But what is its purpose in the body? The scientific community has really just started to figure this out.
Let’s start at the beginning.
Discovered by Tamm and Horsfall at the Rockefeller Institute in 1950, the eponymous substance was initially noted for its striking ability to inhibit antibody crosslinkage of many viruses. By 1952, the pair had published their characterization of the virus inhibitor as a distinct protein in Journal of Experimental Medicine.
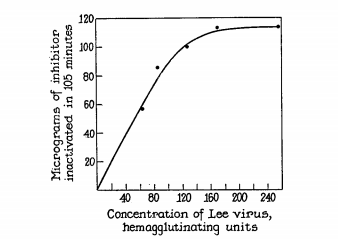
“A mucoprotein, present in normal human urine, has been isolated and obtained in a state of a high degree of purity… it appears that the mucoprotein has a high molecular weight, and is specifically antigenic. This substance, which appears to be free of contaminating material, possesses in extraordinary degree the capacity to react with influenza, mumps, and Newcastle disease viruses.”
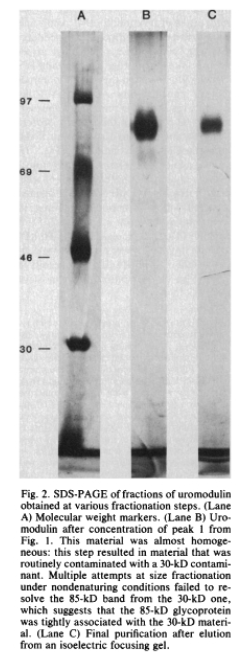
In 1985, several decades and just a few hundred miles away, Muchmore and Decker isolated, purified, and characterized a 85-kd protein which inhibited T-cell proliferation in urine. They named it Uromodulin after its source and activity after further demonstrating it as a high-affinity ligand for IL-1.
But then, just 2 years later, Pennica and associates discovered that uromodulin was, in fact, the same molecule as the well-characterized Tamm Horsfall protein.

Since then, our understanding of uromodulin’s role has deepened. Through a series of experiments, we have learned that uromodulin does just that- modulates the immune response. In the 1990s, experiments showed uromodulin could cause immune system activation,as well as the inhibition described by Muchmore and Decker.
First, in 1990, uromodulin was shown to activate human polymorphonuclear cells and activate the complement pathway in vitro.
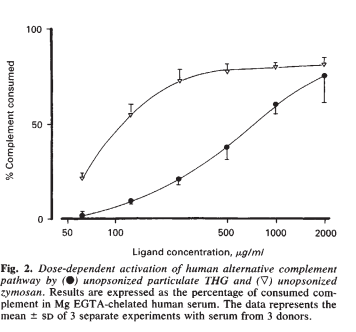
Tamm Horsfall Protein incubated with human serum activated the complement pathway in a dose dependent fashion. (Zymosan is a positive control for complement activation)
Horton et al. 1990
This was refined by experiments showing these activated monocytes in turn secreted tumor necrosis factor (TNF) alpha and other pro-inflammatory chemokines. Subsequent experiments have shown overall protective effect of uromodulin excretion against urinary tract infections.
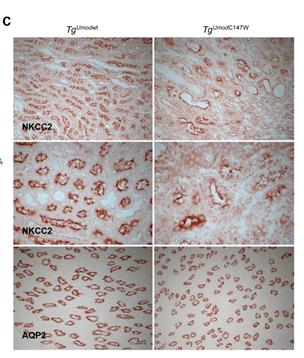
The uromodulin gene was localized to Chromosome 16 in 1993 and sequenced shortly thereafter. Studies characterizing transgenic mouse models have provided useful insight. In 2010, a mouse model was generated expressing a mutant form of uromodulin called UMOD-147 mice. This mutant was derived from a well characterized mutation at amino acid 147 cystine to tryptophan found in patients with autosomal-dominant tubulointerstitial disease. The mouse model utilized the uromodulin promoter to drive production of this mutant version of the protein with faithful expression. The transgene incorporates randomly into the genome of the mouse so both mutant and normal protein is expressed. The investigators inserted a tag in order to distinguish the two proteins. This mutant form of uromodulin was found not to traffic to the apical membrane of the tubular cell. These mice displayed concentrating defect, inflammatory cell infiltration leading to tubulointerstitial fibrosis, and eventually kidney failure.
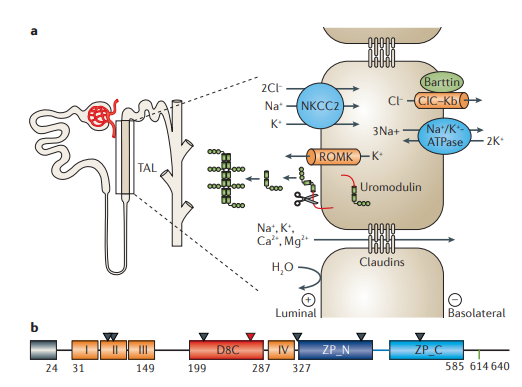
Devuyst et al. 2017
UMOD-147 mice showed downregulation of mRNA and protein abundance of chloride channel Kb (CLCKb), Na-K-Cl contransporter (NKCC2), and the renal outer medullary potassium channel (ROMK) . This sheds light on the role of uromodulin as a regulator of tubule permeability.UMOD-147 mice showed downregulation of mRNA and protein abundance of chloride channel Kb (CLCKb), Na-K-Cl contransporter (NKCC2), and the renal outer medullary potassium channel (ROMK) . This sheds light on the role of uromodulin as a regulator of tubule permeability.
A series of experiments in early 2000 linked uromodulin with the rare medullary cystic kidney disease 2 and familial juvenile hyperuricemic nephropathy. One group of scientists sequenced the DNA of a small group of affected families and found 4 novel mutations in the UMOD gene. In 2003, these mutations were shown to cause decreased Tamm Horsfall Protein excretion and accumulation within tubular cells. Now well into the 21st century, Uromodulin has been studied in an incredible array of disease states. A 2009 Nature Genetics article by Kottgen, et al associated common variants in the UMOD promoter are at risk of chronic kidney disease and hypertension.
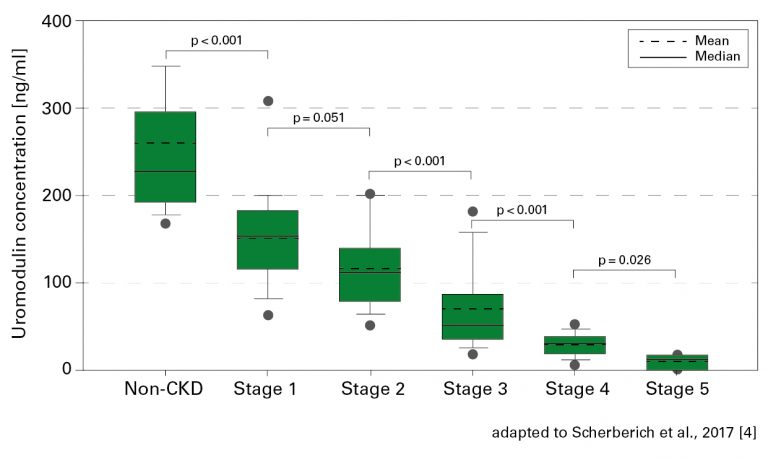
Later, plasma levels of uromodulin were correlated with stages of CKD so tidily that the protein was suggested for use as a biomarker of the disease.
Uromodulin gene expression has been shown to be upregulated in response to ischemic injury in mice. When the gene is knocked out entirely, mice develop more inflammation and tubular necrosis in the face of ischemic injury. This suggests a protective mechanism against the effects of ischemic injury that is encouraged by findings in a 2017 prospective cohort study showing decreased uromodulin levels associated with increased risk of AKI after cardiovascular surgery.
In kidney transplant patients, decreased levels of uromodulin protein in serum also correlate with graft loss and mortality.
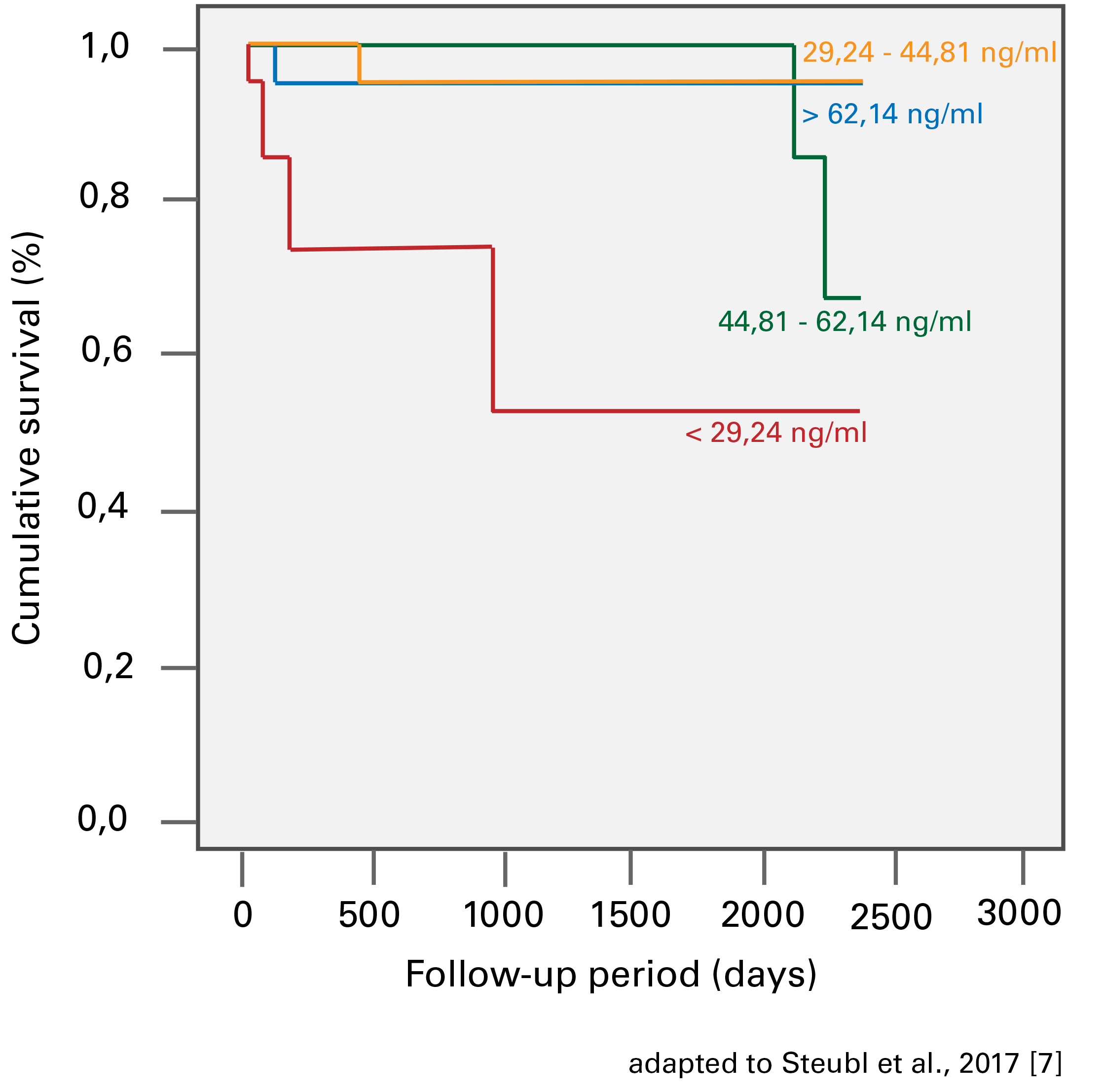
Transplant recipients who had the lowest levels of uromodulin had the lowest rate of survival.
Steubl et al. 2017
These days, there is no shortage of work being done to elucidate the role of uromodulin. Will it be used as a predictor of AKI post operatively? Will it turn out to be, as one expert and author suggests, a ”Guardian of Urinary and Systemic Homeostasis”? Only time (and a lot of science) will tell, but one thing is for sure: we have not seen the last of Tamm and Horsfall’s discovery.

Dr Frank Horsfall, Jr (L) and Dr Igor Tamm. Incidentally not the slightest bit interested in nephrology.
Post by Anna Gaddy, MD @AnnaGaddy



Awesome article! Thanks