Welcome to the 21st case of the Skeleton Key Group, a team of 50-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network. The cases are actual cases (without patient identifying information) that intrigued the treating fellow.
Written by: Priti Meena
Visual Abstract & Infographics: Shweta Shah & Priti Meena
A.The Stem
A 58-year-old man with no known medical history and not on any medications presented to the emergency department with complaints of severe bilateral upper and lower extremity weakness. The weakness was predominantly proximal and evolved in an ascending manner over one day. He does not report any history of vomiting, diarrhea, shortness of breath, loss of consciousness, or chest pain. There was no history of fever, trauma, heavy carbohydrate meal, prior strenuous exercise, or thyroid disease. He has a roughly 30 pack-year smoking history and occasionally consumed alcohol. He lost 8 kg of weight during the last 4 months due to a decreased appetite.
Physical examination
His initial vitals were blood pressure – 170/100 mm Hg, heart rate – 86 bpm, regular, respiratory rate- 16/min, temperature – 97.7°F and SpO2 -97% on room air.
Alert and oriented. Normal superficial/deep sensations and vibration sensations with normal extraocular muscle movements. Deep tendon reflexes were attenuated. Power of bilateral upper and lower limbs was ⅗ symmetrically. Cerebellar signs were normal.
Examination of other systems was unremarkable.
B. The Labs
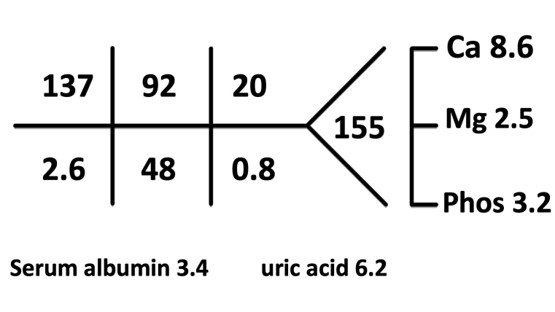
ABG: pH =7.48, HCO3 =35, pCO2 =48, pO2 =82
He was started on intravenous (IV) KCl, amlodipine 10 mg, and spironolactone 100 mg twice a day. Despite a high dose of KCl IV (up to 240 meq/day), his hypokalemia and metabolic alkalosis persisted; however, blood pressure normalized.
C. Differential diagnosis
We have reviewed some aspects of hypokalemia in a previous case. Let’s revise briefly and then go back to our case.
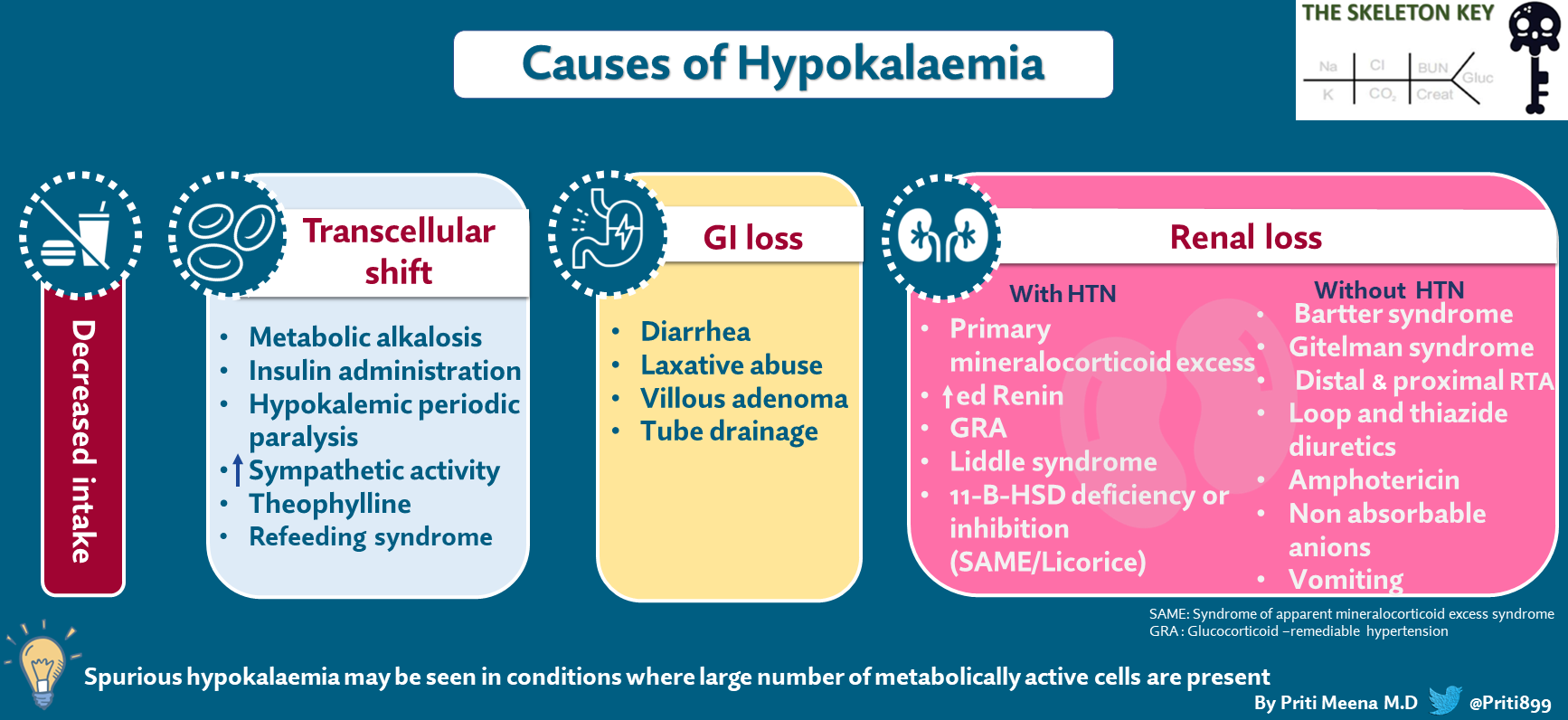
Hypokalemia can be caused either by (Figure 1)
- Insufficient potassium intake
- By excessive potassium loss in the urine or through the GI tract
- Transcellular shift
How do we evaluate this patient further?
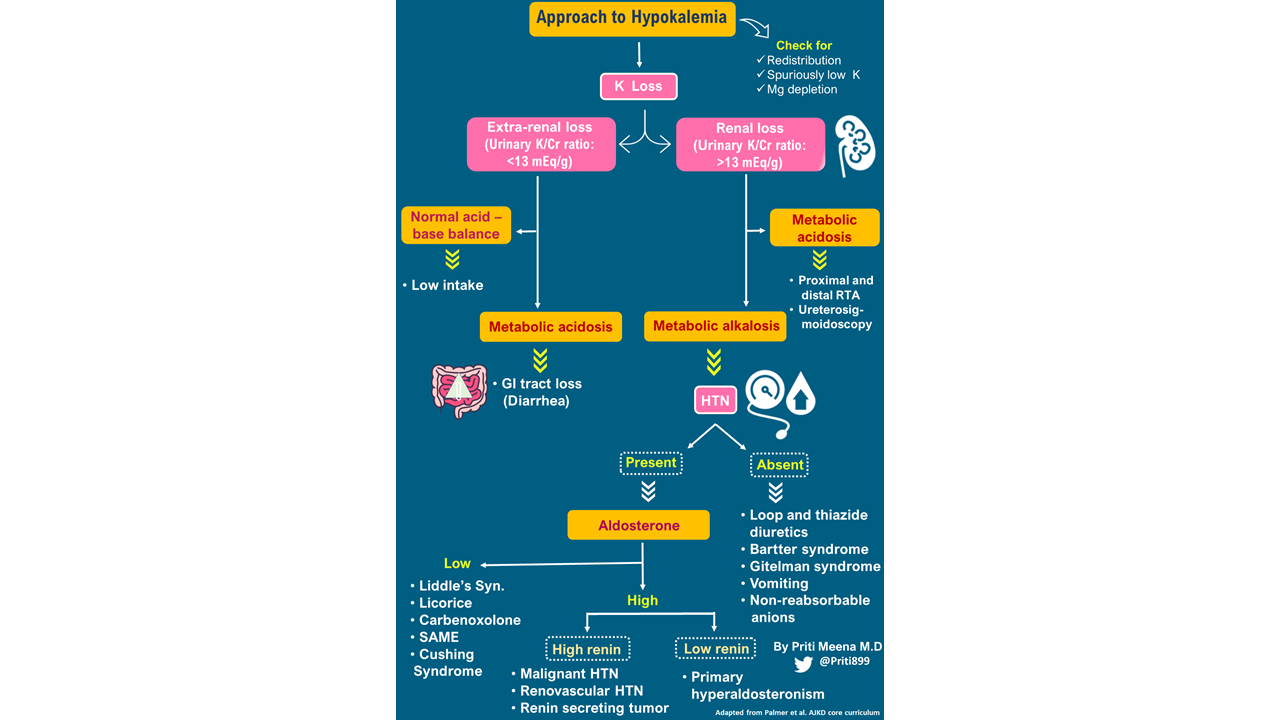
- The etiology of hypokalemia can usually be determined by a careful history and a basic metabolic panel If there is no history suggestive of gastrointestinal losses, or there is no apparent cause, a blood gas analysis and the measurement of urine electrolytes (especially potassium and chloride) can aid in differentiating renal from non-renal loss of potassium.
- In the case of renal losses, the next step is to see if hypokalemia is associated with systemic hypertension or not. In hypertensive hypokalemic patients, the measurement of renin, aldosterone, and cortisol concentrations is helpful in the differential diagnosis.
- Don’t forget to check serum magnesium levels in all the cases of hypokalemia
A simple diagnostic algorithm to approach a patient with hypokalemia is presented in Figure 2.
Our patient had a combination of features. So let’s discuss the differential diagnosis of hypokalemia, metabolic alkalosis, and hypertension (See: Figure 3)
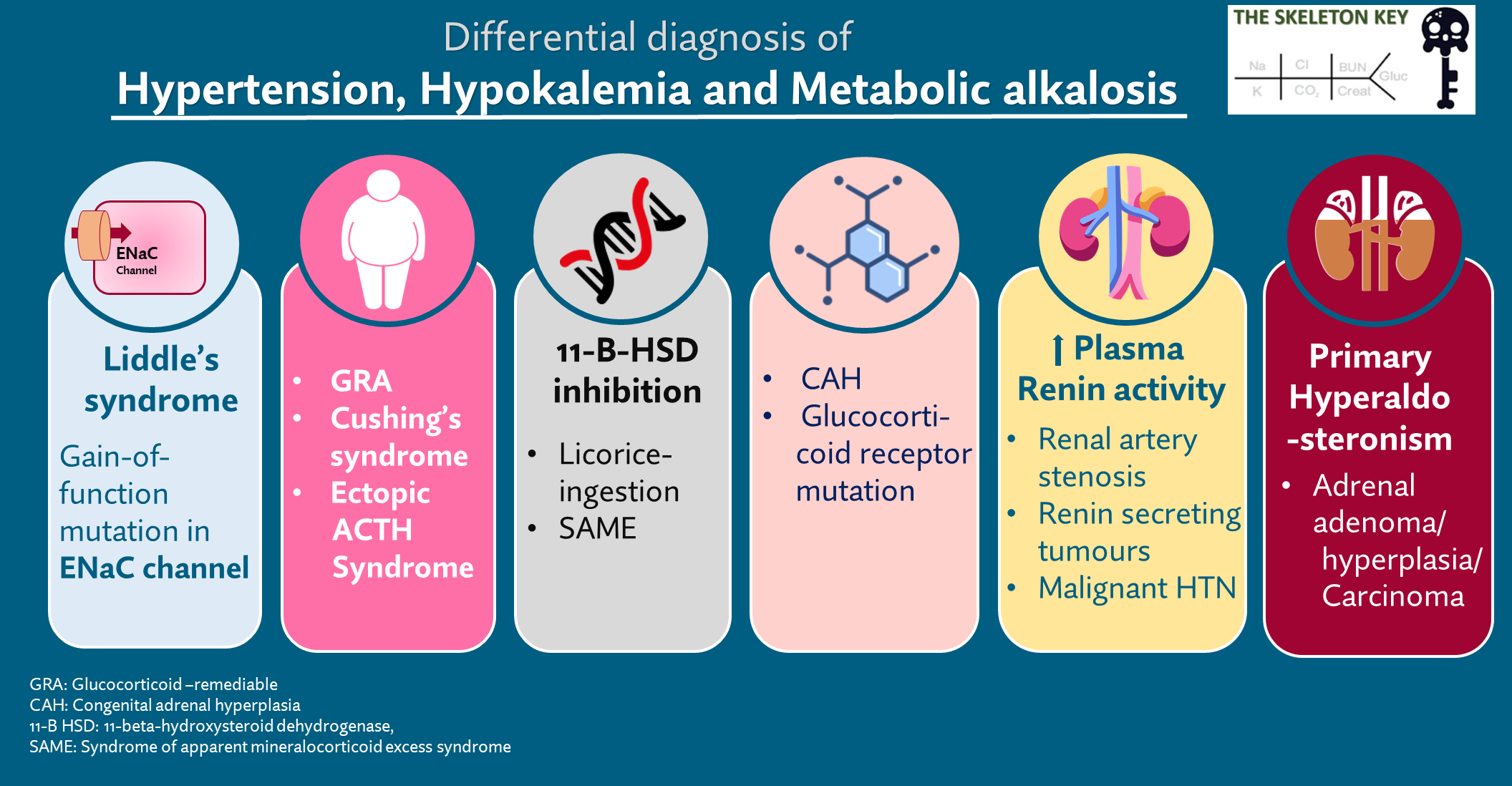
Kidneys are the primary route of potassium elimination from the body and are responsible for excreting approximately 90% of the daily potassium intake. Potassium secretion mainly occurs at the cortical collecting duct (CCD) in the nephron and is mainly mediated by aldosterone. Here potassium secretion is tightly coupled to sodium reabsorption via the epithelial sodium channel (ENaC). Thus whenever there is increased sodium reabsorption via ENaC, potassium secretion increases. Aldosterone plays a pivotal role in regulating blood pressure as well as in maintaining sodium and potassium homeostasis. It binds to the mineralocorticoid receptor (MR), which promotes ENaC activity and thereby increases sodium reabsorption and potassium secretion. Thus any condition such as hyperaldosteronism or Liddle syndrome that increases the activity of ENaC can potentially result in hypokalemia. Additionally, aldosterone can cause metabolic alkalosis by increasing H+ secretion.
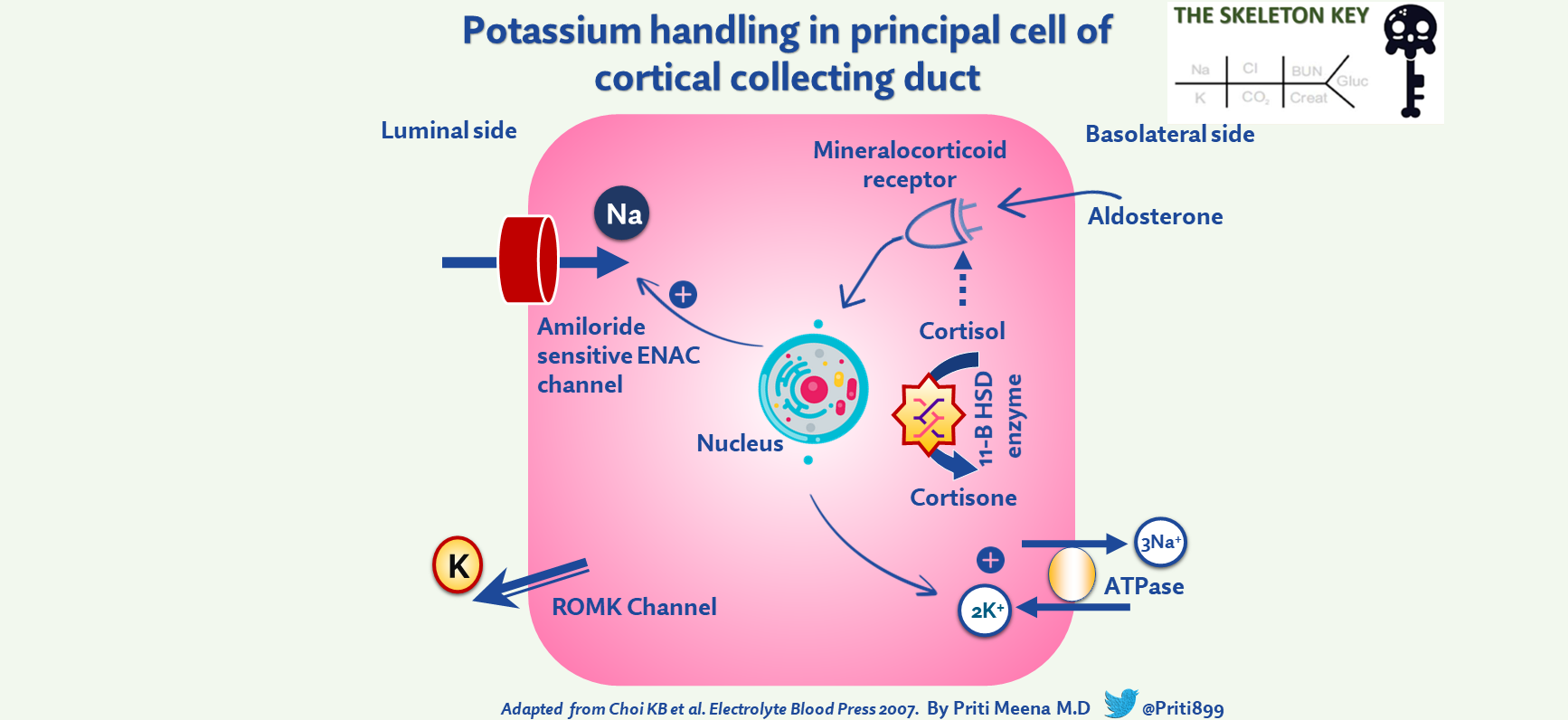
The presence of the clinical triad of hypertension, hypokalemia, and metabolic alkalosis (as in our case) requires evaluation of the renin-angiotensin-aldosterone system which is best approached by measuring plasma renin activity or direct renin concentration and aldosterone concentration.
Chest X-ray showed an undefined nodular opacity in the hilar region of the left lung.
Further evaluation of lung lesion was done in view of a history of smoking, decreased appetite, weight loss.
Chest CT scan showed: A relatively well-defined soft tissue density lesion measuring 2.9×3.4×3.0 cm in the right middle lobe abutting the oblique and horizontal fissure.
A CT-guided biopsy of this lesion was done that demonstrated poorly differentiated small cell lung carcinoma.
D. Final Diagnosis
The patient here presented with a triad of hypokalemia, metabolic alkalosis, and hypertension. These metabolic abnormalities hint towards the diagnosis of mineralocorticoid excess. The patient has low normal plasma aldosterone and direct renin concentration with elevated ACTH and cortisol levels. Together with the patient’s clinical and radiological findings, these findings suggest an ectopic ACTH-dependent Cushing syndrome.
Management
The patient was initiated on ketoconazole, the dose of spironolactone was increased and he was also started on chemotherapy for small cell lung carcinoma after an oncology evaluation.
E. Discussion
DISCUSSION
Cushing syndrome due to ectopic secretion of ACTH accounts for approximately 10% of hypercortisolism cases. Moreover, around half of these cases are associated with small cell lung cancer, and amongst small cell lung cancers, Cushing syndrome occurs in about 5%. Usually, cortisol gets inactivated by conversion to cortisone by 11-beta-hydroxysteroid dehydrogenases (11-β‐HSD2). But cortisol has a high affinity for the mineralocorticoid receptors. However, a very high concentration of cortisol levels may saturate 11-β‐HSD2 at the renal tubules, thereby allowing access of intact cortisol to the renal tubular mineralocorticoid receptor. Thus, hypokalemia and metabolic alkalosis due to glucocorticoids are predominantly due to the ‘spillover’ effect of glucocorticoids onto the mineralocorticoid receptors, which results in enhanced potassium secretion in the cortical collecting duct. Low potassium levels increase the HCO3− reabsorption in Proximal tubules by enhancing sodium bicarbonate cotransporter 1 (NBC-1) expression and activity. Glucocorticoids can also enhance both mRNA expression and functional activity of renal proximal tubule NBC-1 leading to enhanced HCO3− reabsorption in the proximal convoluted tubule in clinical conditions associated with high glucocorticoid production. Differentiation between pituitary and ectopic ACTH secretion-related Cushing syndrome is challenging. A positive response, defined as a decrease in cortisol level to less than 50% of baseline with overnight dexamethasone test, indicates a pituitary-dependent disease; however, failure to suppress suggests adrenal or ectopic tumor production. The degree of suppression in our case was only 8%, consistent with an ectopic source of ACTH secretion. Ectopic secretion of ACTH by tumors like small cell lung carcinomas leads to more pronounced metabolic alterations like hypokalemia and metabolic alkalosis. Tumors that lead to paraneoplastic ACTH secretion are mostly malignant and more advanced than pituitary tumors. They can result in the rapid development of hypercortisolism within a short span, thus classical stigmata of Cushing syndrome such as purple striae and buffalo hump may be absent until the advanced stage. Small cell lung carcinoma associated with ectopic Cushing syndrome often has poor outcomes. In such cases, ketoconazole can be used for the treatment of Cushing syndrome. It acts by inhibiting adrenal glucocorticoid synthesis; however chemotherapy is the standard treatment for small cell lung carcinoma. Since the required dose of ketoconazole in the management of cushing syndrome is very high, it is advisable to closely monitor liver function tests.
F. Take Home Points
- Increased cortisol and Cushing syndrome due to ectopic ACTH secretion is an infrequent cause of hypokalemia. Paraneoplastic syndrome should be strongly suspected in the setting of lung cancer.
- The diagnosis should be considered among patients presenting with metabolic alkalosis and elevated blood pressure. Evaluation includes plasma ACTH and cortisol levels particularly in the presence of lung carcinoma.
- These patients may not necessarily have the characteristic sequelae of chronic corticosteroid excess (Cushingoid phenotype) due to the rapid onset of hypercortisolism.
Post reviewed by Matthew Sparks, Joel Topf, Anna Gaddy, and Sayna Nourouzi



Reminds me of a very similar case dealt as a fellow on a Friday evening a decade ago. The legendary fluid electrolyte guru Prof Kamel Kamel on service helped tease it out on similar lines . Thank you
Excellent case with an even
Better discussion
How do you interpret the urine K/Cr ratio if someone is already replaced with IV KCL at the point of review? Does it skew your urine K/Cr ratio?
Excellent diagrams and fascinating case. Thanks!