Welcome to the second case of the Skeleton Key Group, a team of twenty odd nephrology fellows who work together to build a monthly education package for Renal Fellow Network. The cases are actual cases that intrigued the treating fellow.
Case 2: Written by Juan Pablo Arroyo and Sai Sudha Mannemuddhu
Check out Sayna Norouzi’s video here.
There’s more! A #tweetorial here by Sayna Norouzi.
A. The Stem
A 59 year-old white woman with a history of hypertension and hyperlipidemia, suffered a left femur fracture following a fall. For three days after the fall, she was awake but was unable to move. She was finally saved by her neighbor who found her and brought her to the hospital. During the review of systems she reports poor oral intake for the past couple of months and weight loss of 10 pounds over the last 1-2 months. Her home medications were Carvedilol and Aspirin.
Physical Exam
HR: 95 bpm; BP 110/75 mm of Hg; Sat 95% on 4L NC. BMI 18 Kg/m2 – Weight 47 Kg
She is groggy, but appropriately answers to questions, and appears cachectic. Her neck veins are flat. Lungs are clear to auscultation, heart rate is regular, and there are no murmurs. Abdominal examination is normal. She has no edema.
B. The Labs
Following an open reduction and internal fixation of left femur, post-operative labs were drawn…
| Metabolic Panel | Value |
| Phosphorus | 2.5 mg/dL |
| Calcium | 8 mg/dL |
| Magnesium | 1.9 mg/dL |
| Albumin | 3 g/dL |
Nephrology was consulted for evaluation and management of hypokalemia.
C. Differential Diagnosis: General Approach to Hypokalemia
Is this severe hypokalemia? No, the potassium is > 2.5 mEq/L.
Why is the potassium low? There are 2 important causes of low potassium:
- Increased losses (Gastrointestinal or renal)
- Intracellular shift of Potassium (K)
Low oral intake of potassium can be considered, but it is less likely to cause hypokalemia in the absence of any of the two causes mentioned above. What does the evidence say?
1. Decreased oral intake of potassium
Our patient has the following features consistent with decreased oral intake: low muscle mass, cachectic, ill appearing, recent weight loss, and ketones in the urine.
Healthy kidneys can reduce urinary loss of potassium down to 5 mmol a day (195 mg), so profound hypokalemia would require dietary intake to be south of that. An apple has enough potassium to keep a person in balance. A baked potato has 3 days’ worth. Even if people restrict their diet sufficiently to go into negative potassium balance, any associated cell death (think cell death from starvation) will release intracellular potassium and maintain a normal serum potassium (though they will have depletion of total body potassium). A dramatic demonstration of the rarity of decreased oral intake as the cause of hypokalemia was a study of 1000 outpatients with eating disorders. Only 5% were found to have hypokalemia and all of those had bulimia with either diuretic, cathartic or self-induced vomiting causing increased potassium losses. None of the patients with pure calorie restriction had hypokalemia (despite that cohort having the most severe nutritional deficiency).
2. Increased GI or kidney loss of potassium
Check for a history of GI losses (diarrhea, vomiting, feeding tube output, ostomy output etc). Our patient did not have any of the above.
Furthermore, lower GI or kidney losses can be differentiated by looking at urine electrolytes and calculating the potassium losses in urine by Fractional excretion of Potassium (FeK), and Urine potassium-to-creatinine ratio (K/Cr). (discussed in detail below). It is important to note that upper GI losses would look like kidney losses.
3. Shift of potassium into cells
This usually causes only transient drops in potassium, though more prolonged and symptomatic hypokalemia can occur with hypokalemia periodic paralysis (thought to be an abnormal calcium channel?), barium toxicity causes increased activity of the Na-K-ATPase and if there is total body and intracellular depletion of potassium. Metabolic alkalosis could cause hypokalemia due to an intracellular shift but most of the drop in potassium is from increased urinary losses of potassium (identified by urine electrolytes measurement) which tends to occur with metabolic alkalosis. Here is a nice case report of recurrent metabolic alkalosis in a dialysis patient (so kidney losses can not contribute to hypokalemia) where metabolic alkalosis was associated with repeated hypokalemia. Prolonged and profound increases in insulin or catecholamines could also cause hypokalemia.
What did our patient’s urine electrolytes show?
K: 10 mmol/L. Cr: 80 mg/dL (7.07 mmol/L). Na: 20 mmol/L
- TTKG was not calculated due to its limitations.
- Fractional excretion of Potassium (FeK): 3.3%, which is appropriate conservation for a hypokalemic patient. Range in normal patients is 4-16%, in patients with hypokalemia of extrarenal origin it is 1.5%-6.4% and in hypokalemia of renal origin it is 9.5-24%. However, FeK has its limitations as well. Since all the potassium in urine is secreted in the late distal tubule and medullary collecting duct, it is not a true fractional excretion.
- Urine potassium-to-creatinine ratio (K/Cr) is another way to differentiate renal vs extra renal losses. Normal values are < 1.5 mmol/mmol (13 mEq/g) of creatinine. Pt had K/Cr: 1.4 mmol/mmol. This is appropriate conservation. In the absence of diarrhea, this can point towards intracellular shifts. However, a similar picture can be seen after prolonged diuretic usage.
D. More Data
Next set of labs ~ 8 hrs later while on 75 mL/hr of Ringers lactate. Additionally, our patient was started on a regular diet and received oral potassium of 60 mEq.
E. The Answer
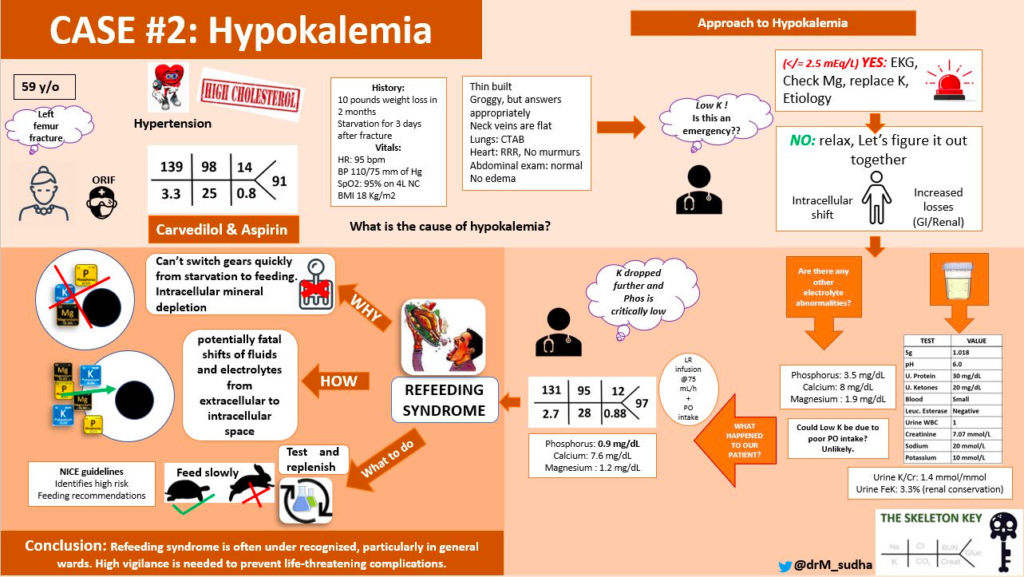
Why did the potassium drop despite receiving supplement? How are her Ca, Mg, and Phosphorus now?
- Once the patient started eating, serum potassium, magnesium, calcium, and phosphorus (critically low) dropped. See the following table:
We have a case of refeeding syndrome here!
Refeeding syndrome (RFS) can be defined as a potentially fatal shift in fluids and electrolytes that may occur in malnourished patients receiving refeeding (whether enterally or parenterally). During a period of starvation patients lose electrolytes, though blood levels remain normal. This leaves a patient at risk for refeeding syndrome as the body is already depleted. Then when the fast ends and refeeding begins insulin causes a rapid shift of extracellular potassium, phosphorus, magnesium, and of course, glucose, into the cells. This unmasks the underlying electrolyte deficiencies and can expose the patients to life threatening electrolyte disturbances. Hypophosphatemia is a prominent finding in refeeding syndrome. In this Dutch study of 178 patients admitted to a medicine service, 54% were considered to be “at-risk” of RFS. Fourteen percent of them actually developed RFS, (8% of the entire study cohort). Awareness of refeeding syndrome crystallized in 1981 with the publication of a case report of two fatal episodes following the institution of parenteral nutrition in profoundly malnourished patients. The paper was republished in 2008 with commentary. It’s worth a read.
More commonly seen in cancer patients with cachexia, and in ICUs due to hyperalimentation. It is under recognized in patients admitted to general wards, like this patient.
NICE guidelines criteria to identify high risk patients for refeeding syndrome:
- Either the patient has 1 or more of the following:
- Body mass index (kg/m2) <16
- Unintentional weight loss >15% in the past three to six months
- Little or no nutritional intake for >10 days
- Low levels of potassium, phosphate, or magnesium before feeding
- OR the patient has 2 or more of the following:
- Body mass index <18.5
- Unintentional weight loss >10% in the past three to six months
- Little or no nutritional intake for >5 days
- History of alcohol misuse or drugs, including insulin, chemotherapy, antacids, or diuretics
Our patient fits in the high-risk category. Being elderly is another risk factor that is not mentioned here as up to 50% of older people have a high risk for malnutrition. Studies show that low caloric intake results in lower mortality rates in critically ill RFS patients compared with RFS patients on full nutritional support.
You can’t have a blog post about refeeding syndrome without sharing this letter to the NEJM where the Magician David Blaine suffered refeeding syndrome following a stunt that included a 44-day fast.
Now management…
NICE recommends limiting nutrition to 10 KCal/Kg/d for patients at high risk for RFS. Here is the algorithm adapted from NICE guidelines for the management of malnourished patients.
Our patient was managed with potassium, phosphorus, and magnesium supplementation, along with slow feeding. She received 40 mEq of IV potassium, followed by 40 mEq oral, twice a day for 3 days, 2 g of magnesium sulfate IV every 12 hours for 2 days, and 2 doses of 40 mmol of IV phosphorus, followed by 16 mmol oral, twice a day for 3 days.
Take Home Message
- Suspect refeeding syndrome when there is hypokalemia in the setting of starvation and weight loss followed by nutritional supplement.
- Identify high risk patients
- Serial measurement of K, Phos, Mg and Ca can aid in diagnosis.
- Urine electrolytes will help in teasing out the cause of hypokalemia.
- NICE guidelines are helpful for feeding recommendations. Slow feeding prevents rapid shifts of electrolytes from ECF to ICF.
- Replenish electrolytes, and vitamins appropriately along with macro nutrients.



Thank you for the nice analysis. Had a query in the schema @medrants @CPSolvers. Shouldn’t the Urine K/Cr ratio be 1.5 instead of 20 (a typo probably)
I can send you PDF of the case if you like. let me know your email address
Excellent analysis. So many confusion, I have cleared from this.How can I get PDF of this topics.