Welcome to the 30th case of the Skeleton Key Group, a team of 50-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network. These are actual cases (without patient identifying information) that intrigued the treating physician.
Written by: Santhoshi Bavi, MD
Visual abstract by: Shweta Shah, MD
A. A Tale of Three Admissions
Admission 1:
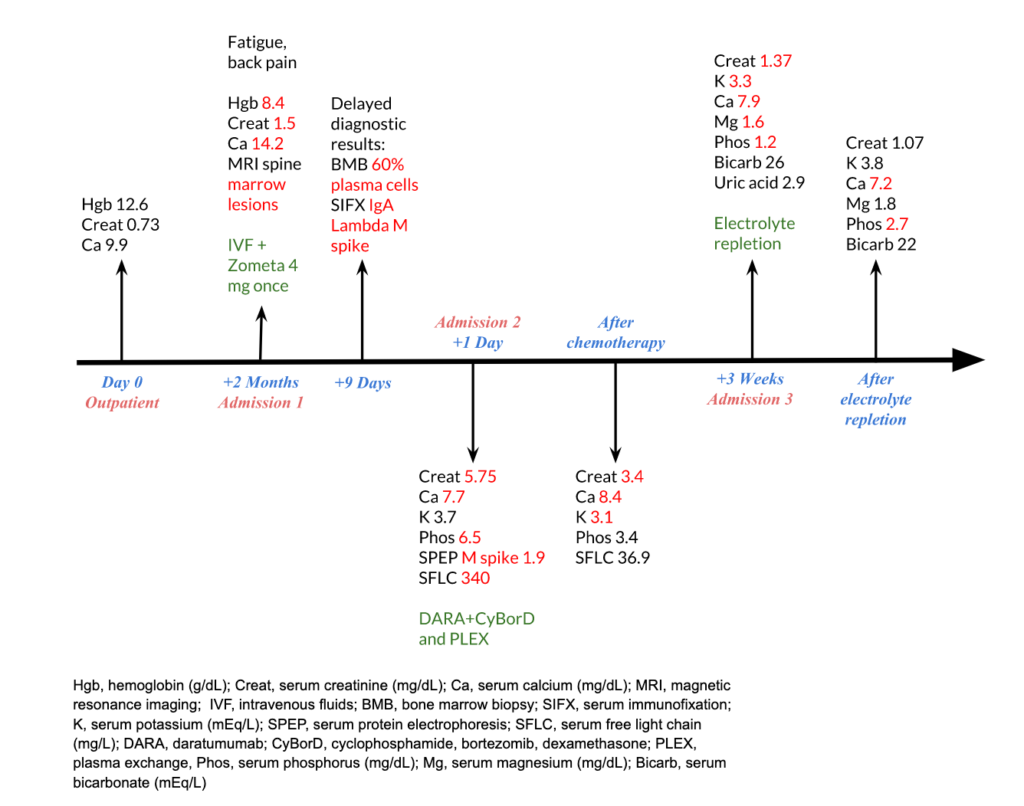
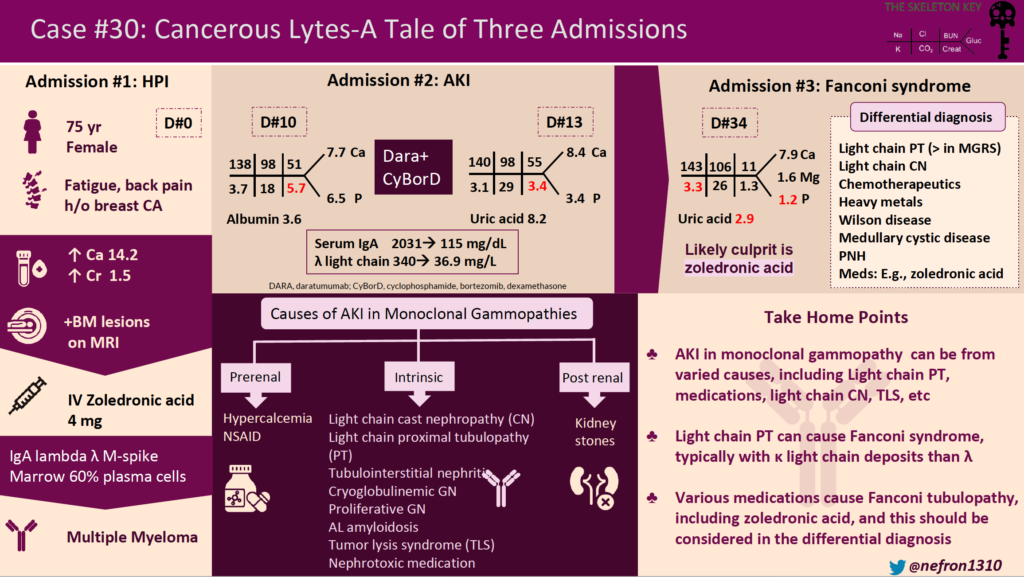
A 75-year-old woman with a remote history of breast cancer presented with fatigue and back pain and was admitted for the first of three admissions. Diagnostic workup revealed hypercalcemia (14.2 mg/dL), acute kidney injury (serum creatinine 1.5 mg/dL with a previous baseline of 0.7 mg/dL), anemia (hemoglobin of 8.4 g/dL), and marrow infiltrating lesions on MRI spine. She received intravenous fluids and a dose of zoledronic acid, with improvement in serum calcium and kidney function. Serum immunofixation revealed an IgA lambda M spike. Bone marrow biopsy showed 60% plasma cells, confirming the diagnosis of multiple myeloma. She was discharged with a serum creatinine of 1.39 mg/dL.
Admission 2:
Ten days later, she was hospitalized for worsening acute kidney injury. She had symptoms of fatigue and myalgias. She had no nausea, vomiting, diarrhea, or rash. On presentation, her heart rate was 67/min, and blood pressure was 121/58 mm Hg. She was euvolemic on the physical exam. Relevant labs were as follows:
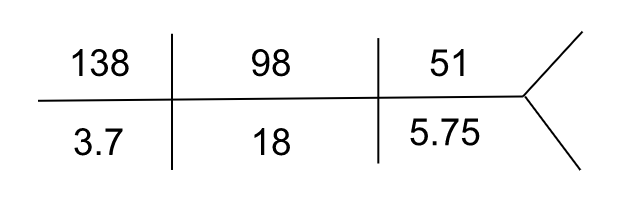
| Serum calcium | 7.7 mg/dL |
| Serum phosphorus | 6.5 mg/dL |
| Serum albumin | 3.6 g/dL |
| Hemoglobin | 9 g/dL |
| Urinalysis | blood, protein 50 mg/dL |
| FeUrea | 20 % |
| Serum protein electrophoresis | M spike 1.9 g/dL |
| Serum free light chain assay | Lambda light chains 340 mg/L (reference range: 5.7 – 26.3 mg/L)Kappa light chains 13.2 mg/L (reference range: 3.3 – 19.4 mg/L)Lambda to kappa ratio 25.7 |
| Serum IgA level | 2031 mg/dL |
She was presumed to have light chain cast nephropathy and was treated with daratumumab, cyclophosphamide, bortezomib, and dexamethasone (Dara-CyBorD).
Repeat labs three days after treatment:
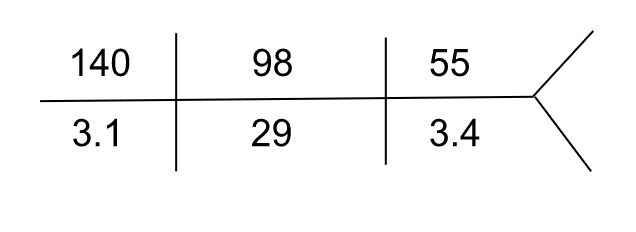
| Serum calcium | 8.4 mg/dL |
| Serum phosphorus | 3.4 mg/dL |
| Serum uric acid | 8.2 mg/dL |
Serum free light chain assay showed a reduction in lambda light chain level to 36.9 mg/L
Serum IgA level was reduced to 115 mg/dL
STOP! Let’s discuss the differentials for AKI in monoclonal gammopathy.
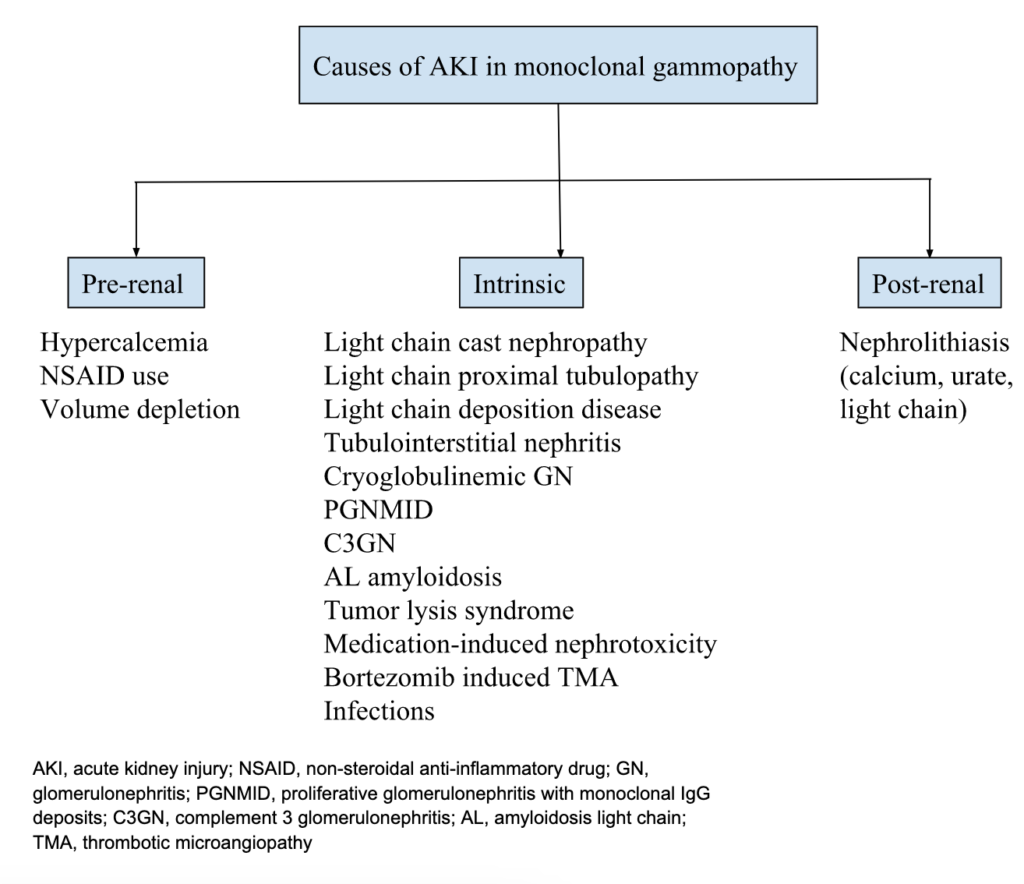
Important causes of acute kidney injury to consider in patients with monoclonal gammopathy are light chain cast nephropathy, light chain proximal tubulopathy (LCPT), AL amyloidosis, tumor lysis syndrome, hypercalcemia, and medication-induced nephrotoxicity. Light chain cast nephropathy is a myeloma-defining event and is diagnostic of a hematological malignancy.
Light chain cast nephropathy refers to tubular obstruction and damage caused by the formation of light chain casts resulting in acute kidney injury. This patient was presumed to have light chain cast nephropathy, hence was treated with chemotherapy. Cast nephropathy often has a precipitating factor such as dehydration, hypercalcemia, NSAID use, etc. This patient had hypercalcemia which could have acted as a precipitating factor. Proteinuria is the presenting feature in all patients with cast nephropathy, and 10% of patients have nephrotic-range proteinuria. The risk for cast nephropathy is associated with the level of light-chain excretion. International Myeloma Working Group’s (IMWG) threshold of serum free light chain level for developing cast nephropathy is >1500 mg/L whereas International Kidney and Monoclonal Gammopathy (IKMG) research group’s threshold is >500 mg/L. This patient’s serum free light chain level (340 mg/L) was below both of these thresholds. Light chain cast nephropathy with this serum free light chain level would be uncommon and a kidney biopsy would be required to make the diagnosis. Kidney biopsy would not result in a change in management but it has prognostic value in myeloma cast nephropathy. Had the patient had much higher serum free light chains, the expert opinion does recommend chemotherapy and plasmapheresis.
Light chain proximal tubulopathy (LCPT) is predominantly seen in patients with kappa-restricted disease and is more common in monoclonal gammopathy of renal significance (MGRS). Hence, it is an unlikely cause of kidney injury in this patient with multiple myeloma and lambda light chain predominance.
Tumor lysis syndrome (TLS) is commonly seen during the therapy of bulky, rapidly proliferating tumors. Leukemia and lymphoma are the most common malignancies associated with tumor lysis syndrome. Multiple myeloma is a low-proliferation tumor with a rare incidence of TLS. But an increase in the incidence of TLS has been noted after introducing bortezomib in the therapy of multiple myeloma. Bortezomib is a proteasome inhibitor that has been reported to induce TLS in patients with multiple myeloma. This patient developed hyperuricemia and hyperphosphatemia after treatment with bortezomib which raises suspicion of TLS being one of the contributors to renal injury. Hypokalemia is very unusual with TLS although it is likely explained by urinary potassium loss from proximal tubular dysfunction in this patient (as described below).
Hypercalcemia in this patient may have initially contributed to kidney injury and acted as a precipitating factor for cast nephropathy. But she developed worsening kidney function while the serum calcium normalized, thus eliminating hypercalcemia as the cause of the AKI on this admission.
Nephrotoxic chemotherapeutic agents. This patient was treated with daratumumab, cyclophosphamide, and bortezomib which have no known nephrotoxic potential, although AKI due to thrombotic microangiopathy (TMA) from bortezomib is an important differential to consider.
Finally, zoledronic acid could also have contributed to kidney injury in this patient. It is excreted almost entirely by the kidney, hence reduction in kidney clearance can lead to its accumulation and kidney toxicity. It can induce nephrotoxicity by dysregulating fatty acid metabolism in tubular epithelial cells. It is associated with acute tubular necrosis, tubulointerstitial nephritis, collapsing focal segmental glomerulosclerosis, and interstitial fibrosis tubular atrophy (IFTA). However, it was found to be safe in patients with normal creatinine clearance at baseline.
Check out the following links to learn more about AKI in multiple myeloma and MGRS:
- The evaluation of monoclonal gammopathy of renal significance: A consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nature Reviews Nephrology, 15(1), 45-59.
- Monoclonal gammopathy of renal significance. New England Journal of Medicine, 384(20), 1931-1941.
- DDx for Acute Kidney Injury in Myeloma Patients. Renal Fellow Network.
Admission 3:
She was hospitalized again 3 weeks later for worsening fatigue and grossly deranged electrolytes as follows. She did not have any nausea, vomiting, or diarrhea and she was hemodynamically stable.
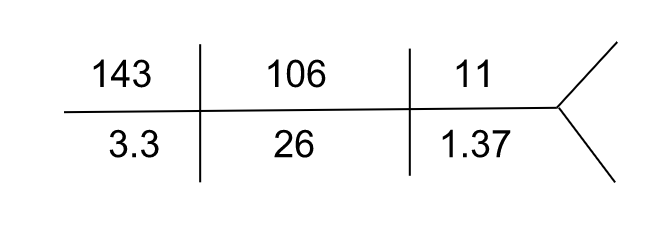
| Serum anion gap (corrected for albumin) | 10 mEq/L |
| Serum calcium | 7.9 mg/dL (ionized – 3.7 mg/dL) |
| Serum magnesium | 1.6 mg/dL |
| Serum phosphorus | 1.2 mg/dL |
| Serum uric acid | 2.9 mg/dL |
Urinalysis showed no glucosuria, pyuria, or hematuria, and urine pH was 4.8. Due to gross derangement of electrolytes in this patient with multiple myeloma, proximal tubular dysfunction was suspected and a quantitative urine assay for amino acids was done which was positive.
What are your differentials for the above electrolyte abnormalities namely hypokalemia, hypophosphatemia, and hypomagnesemia?
Fanconi syndrome caused by light-chain proximal tubulopathy, or medication-induced tubular toxicity
Gastrointestinal loss of electrolytes from colitis, etc. (but the patient did not have diarrhea)
Poor oral intake
Diuretics
After electrolyte repletion, her labs were:

| Serum anion gap | 9 mEq/L |
| Serum calcium | 7.2 mg/dL |
| Serum magnesium | 1.8 mg/dL |
| Serum phosphorus | 2.7 mg/dL |
TIMELINE:
B. The Diagnosis
This 75-year-old woman with multiple myeloma presents with hypokalemia, hypophosphatemia, acute kidney injury, low normal serum uric acid (despite recent chemotherapy and acute kidney injury), and positive urine amino acid. This clinical picture is suggestive of Fanconi syndrome.
Fanconi syndrome is characterized by proximal tubular dysfunction resulting in the inability to reabsorb electrolytes and other substances. This results in glucosuria, phosphaturia, hypophosphatemia, hypokalemia, uricosuria, hypouricemia, generalized aminoaciduria, and proximal (type II) renal tubular acidosis. This patient’s serum uric acid was low normal even though she recently received chemotherapy which increases the risk of hyperuricemia, especially in acute kidney injury, which suggests that there was uricosuria.
One perplexing part of this story is the normal serum bicarbonate level. Fanconi syndrome typically causes normal anion gap metabolic acidosis (NAGMA) as it causes proximal RTA. The absence of this finding in this patient is difficult to fully characterize without additional data including urine pH, ABG, and urine bicarbonate.
What caused Fanconi syndrome in this patient?
A common cause of Fanconi syndrome in monoclonal gammopathies is light-chain proximal tubulopathy (LCPT), which is characterized by cytoplasmic inclusions of monoclonal light chains within proximal tubular cells. At least 30% of patients with LCPT present with Fanconi syndrome. But light chain proximal tubulopathy rarely occurs in multiple myeloma, it is more commonly present in the MGRS stage. LCPT is predominantly crystalline and Kappa-restricted, whereas this patient had Lambda light chains. This patient was presumed to have cast nephropathy which is a myeloma-defining event, making LCPT less likely the cause of Fanconi syndrome in this patient.
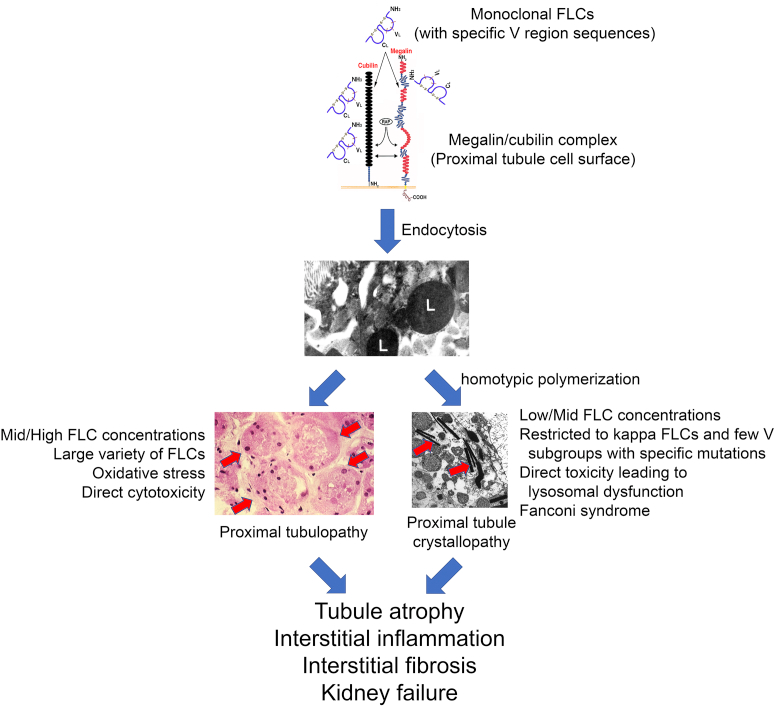
Figure 1. Proximal tubule toxicity of free light chains. Sirac et al. Kidney Int Rep 2021
Another possible cause of Fanconi syndrome is tubular toxicity caused by chemotherapeutic agents. Cisplatin and ifosfamide are the most commonly implicated drugs, which this patient did not receive. Although cyclophosphamide is a structural isomer of ifosfamide, it is not known to be nephrotoxic. Bortezomib can cause kidney injury through thrombotic microangiopathy (TMA).
Some other differentials for Fanconi syndrome are primary and secondary hyperparathyroidism, medullary cystic disease, paroxysmal nocturnal hemoglobinuria, Wilson’s disease, drugs and toxins including outdated tetracycline, gentamicin, lead, cadmium, mercury, etc, which were not suspected in this case.
Hence, the hypothesis is that Fanconi syndrome in this patient was likely caused by zoledronic acid that was given to treat hypercalcemia. Zoledronic acid is nephrotoxic and there are several case reports of zoledronic acid-associated Fanconi syndrome. Hypokalemia, hypophosphatemia, and kidney dysfunction are the most common findings in these cases. The time of onset of Fanconi syndrome after initiation of zoledronic acid varies widely, ranging from days to weeks to months after exposure. In this case, it occurred about a month after exposure to zoledronic acid. There is no established relation yet between the dosage of zoledronic acid and the incidence of Fanconi syndrome. However, cytotoxic effects were only seen at higher administration doses in invitro studies. Zoledronic acid treatment in mouse models showed that defective fatty-acid beta-oxidation and elevated lipid accumulation contributed to its mechanism of nephrotoxicity. Renal biopsy available in a limited number of cases showed acute tubular necrosis, tubulointerstitial nephritis, interstitial fibrosis and tubular atrophy (IFTA). In most cases, Fanconi syndrome was reversible after stopping the zoledronic acid on short-term follow-up.
Some shortcomings of these case reports are a lack of long-term follow-up data, lack of data to determine risk factors for developing Fanconi syndrome such as low initial eGFR, etc., and a lack of kidney biopsy to define the pathology in most cases.
C. Take-home messages
- The differential diagnosis of acute kidney injury in a patient with monoclonal gammopathy includes hypercalcemia, light chain cast nephropathy, light chain proximal tubulopathy, tumor lysis syndrome, AL amyloidosis, and nephrotoxic medications.
- Consider Fanconi syndrome in a patient with monoclonal gammopathy and hypokalemia, hypophosphatemia, hypouricemia, and metabolic acidosis.
- Light chain proximal tubulopathy (LCPT) is a common cause of Fanconi syndrome in monoclonal gammopathies.
- LCPT is usually caused by kappa light chains and occurs commonly in MGRS and rarely coincides with multiple myeloma.
- Fanconi syndrome can be caused by drugs & toxins such as outdated tetracycline, gentamicin, ifosfamide, lead, cadmium, mercury, etc. In this case, we also considered zoledronic acid as a possible trigger of the disease.
Reviewed by: Raad Chowdhury, Jefferson L Triozzi, Shweta Shah, Joel Topf, Isabelle Dominique Tomacruz, Sacha Moore, Chi Chu, Matthew A. Sparks, Margaret DeOliveira


