Welcome to the 6th case of the Skeleton Key Group, a team of twenty odd nephrology fellows who work together to build a monthly education package for Renal Fellow Network. The cases are actual cases (without patient identifying information) that intrigued the treating fellow.
Written by Dhwanil Patel
Visual Abstract: Kartik Kalra
A. The Stem
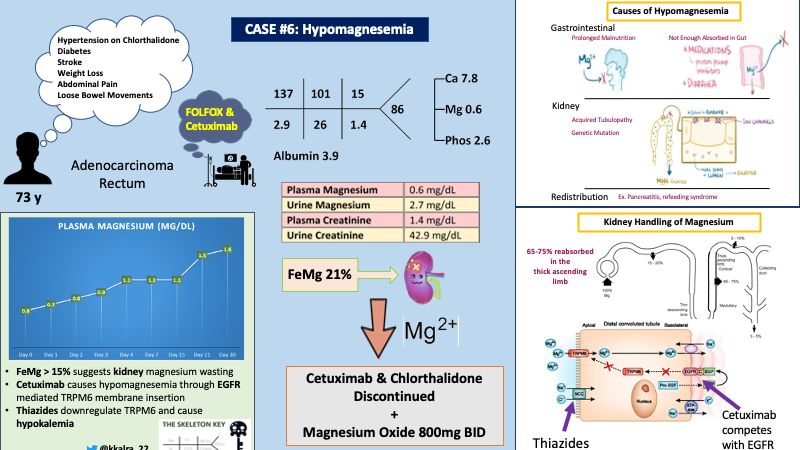
A 73 year old male with past medical history of hypertension, diabetes, ischemic stroke was diagnosed with rectal adenocarcinoma by oncology when he initially presented 3 months ago with a 20 pound unintentional weight loss over the course of a year, along with crampy abdominal pain and loose bowel movements for several weeks. Patient was started on FOLFOX (folinic acid, fluorouracil and oxaliplatin) after initial diagnosis and cetuximab was added one month ago due to metastatic disease.
On follow-up today with oncology, labs were obtained as shown below, prompting referral to nephrology for evaluation of hypomagnesemia. At this time, he reported a normal appetite, regular bowel movements, and improved abdominal pain.
His medications included atenolol 50mg daily, chlorthalidone 25mg, nifedipine 90mg, sitagliptin 50mg, loperamide as needed for diarrhea, and ondansetron as needed for nausea.
B. The Labs
Baseline Cr 0.9 mg/dL, 3 months ago, prior to initiation of chemotherapy.
C. Differential Diagnosis: Hypomagnesemia
When thinking about hypomagnesemia it is useful to divide the etiologies into three buckets: poor magnesium absorption, magnesium wasting nephropathies, and movement of magnesium from the serum into insoluble compartments and compounds.
Differential Diagnosis for Hypomagnesemia ([Mg] 1.6mg/dL):
- Gastrointestinal (GI)
- Reduced intestinal absorption/intake (proton pump inhibitors)
- Increased losses (diarrhea, prolonged nasogastric suction)
- Magnesium wasting nephropathies (tubular defects)
- Acquired Tubular Defects
- Polyuric phase of acute tubular necrosis
- Alcohol induced tubular dysfunction
- Drug induced [loop/thiazide diuretics, epidermal growth factors receptor (EGFR) inhibitors (e.g. cetuximab), aminoglycosides, calcineurin inhibitors, pentamidine, amphotericin b)
- Genetic tubular defects due to mutations of transporters related to handling of magnesium
- Gitelman syndrome
- Mutation in SLC12A3 gene coding for sodium-chloride cotransporter (NCC) in the distal convoluted tubule, leads to decreased expression of Transient Receptor Potential Melastatin type 6 (TRPM6) channel.
- Familial hypomagnesemia with hypercalciuria with nephrocalcinosis
- Mutation in CLDN16 or CLDN19 genes coding for claudins 16/19, which are involved in paracellular absorption of magnesium
- Mutation in CLDN16 or CLDN19 genes coding for claudins 16/19, which are involved in paracellular absorption of magnesium
- Hypomagnesemia with secondary hypocalcemia
- Mutation in TRPM6 gene coding for TRPM6 channel leads to profound hypomagnesemia with secondary hypocalcemia due to impaired parathyroid hormone (PTH) production.
- Compartmental redistribution
- Pancreatitis: In acute pancreatitis, lipase metabolizes triglycerides to free fatty acids. The fatty acids are capped by a carboxyl group with a charge of -1. The Carboxyl group binds cations, specifically Mg and Ca. The carboxyl-Mg (and carboxyl-Ca) complex precipitates out of solution lowering the plasma magnesium (and calcium). This is called saponification.
- Transcellular shifts of magnesium into cells
- Epinephrine
- Hungry bone syndrome
- After parathyroidectomy, magnesium, potassium, phosphorus, and calcium concentrations all drop as osteoid is rapidly remineralized with the sudden drop in PTH.
- Refeeding syndrome
D. More Data
E. Working Through the Differential Diagnosis
Compartmental redistribution is unlikely based on the patient’s history. The next step is to differentiate between magnesium wasting nephropathy versus gastrointestinal etiologies by calculating the fractional excretion of magnesium.
In the presence of hypomagnesemia, the normal kidney response is to decrease magnesium excretion. As depicted in the figure below, there is a sharp decline in urine magnesium excretion (red line) in response to dropping plasma magnesium concentration (blue line).
Fractional Excretion of magnesium (FeMg)
Plasma magnesium in the denominator is multiplied by 0.7 to calculate the amount of plasma magnesium that is actually filtered. This is because 30% of magnesium is protein bound. In the presence of hypomagnesemia, urinary excretion of magnesium is expected to be less than ~<24 mg per day or FEMg less than 4%. Elisaf et al showed that, patients with hypomagnesemia with magnesium wasting nephropathy had average FEMg of 15% (range 4-48%).
Using the values from the table above, let’s calculate the FeMg for our patient:
FeMg of 21% in the setting of hypomagnesemia strongly suggests this patient has a magnesium wasting nephropathy…
The kidneys play an important role in maintaining magnesium homeostasis. Approximately 2400mg of Mg is filtered daily by the glomerulus, of which approximately 90-95% is reabsorbed. Only about ~100mg or <4% is excreted in the urine. Interestingly, in states of hypo- or hypermagnesemia, urinary excretion can vary significantly from 0.5% to 70% of filtered load.
Unlike other electrolytes, only about 10-25% of Mg is absorbed in the proximal tubule. The majority (60-70%) of the filtered Mg is reabsorbed in the thick ascending limb by passive paracellular transport of Mg via channels called Claudins 16 and 19 (refer to image below). This paracellular transport is mediated by a lumen positive potential in the thick ascending limb, which is generated by NKCC2 on the apical side and Na/K ATPase and ClC-Kb channel on the basolateral side.
The remainder of Mg ~10% is reabsorbed in the distal convoluted tubule by active transcellular process with TRPM6. TRPM6 expression on the apical membrane is regulated by epidermal growth factor.
In our patient, cetuximab and chlorthalidone were the most likely culprits per review of his medications associated with urinary magnesium wasting. His acute kidney injury was attributed to volume depletion.
Cetuximab has been associated with hypomagnesemia in ~58% of cases. By competing with epidermal growth factor for its receptor (EGFR), cetuximab decreases expression of TRPM6 on the apical membrane, leading to increased urinary magnesium (see figure below).
Chronic use of thiazides also leads to decreased expression of TRPM6, independent of the EGFR. This is similar to the cause of hypomagnesemia in Gitelman syndrome. Hypokalemia, in the setting of chronic thiazide use, has also been suggested to lead to impaired Mg transport by impairing membrane voltage potential.
Cetuximab and chlorthalidone were discontinued, and the patient was started on magnesium oxide 800mg twice a day. The graph below shows our patient’s magnesium trend over the course of a month after discontinuation of cetuximab.
What is the cause for our patient’s hypokalemia and hypocalcemia?
Hypomagnesemia leads to urinary potassium wasting. Potassium secretion from the distal convoluted tubular cell into the lumen is mediated by the ROMK channel. This process is inhibited intracellular magnesium, as shown in figure below. With hypomagnesemia and low intracellular magnesium, there is a decreased inhibitory effect on potassium efflux from the cell to luminal side (refer to the image below). With intracellular K concentration higher than extracellular, this gradient leads to excretion of K through the ROMK channels (which are not inhibited by low magnesium levels).
Hypokalemia in this setting is refractory to potassium supplementation and requires correction of magnesium deficit to suppress ROMK induced K excretion.
Hypocalcemia in patients with hypomagnesemia is contributed by hypoparathyroidism, PTH hormone resistance and vitamin D deficiency. Low magnesium levels decrease PTH secretion. PTH stimulates cyclic adenosine monophosphate (cAMP) generation. However, in patients with magnesium depletion, it is hypothesized that cAMP generation is impaired leading to PTH resistance. Replacing magnesium causes a rise in PTH levels and increases the response to PTH. Another contributing factor of hypocalcemia is low calcitriol levels.
F. A Few More Words on Magnesium
Magnesium, the second most abundant intracellular cation (after potassium), is important for enzymatic and physiological processes in the human body, such as glycolysis, DNA synthesis/transcription, and adenosine triphosphate (ATP) generating reactions.
Ninety-nine percent of total body magnesium is intracellular, primarily in the bones and skeletal muscles. The remaining 1% of magnesium is found in the extracellular compartment and exists as either free (ionized) magnesium which is biochemically active; or is protein bound or complexed with anions such as chloride, phosphate, and bicarbonate. Protein bound and complexed magnesium is biochemically inert. Homeostasis is maintained by GI tract, bones and the kidneys.
Here is a graphic to show the distribution of magnesium in the body:
Hypomagnesemia is found in about 7-11% of hospitalized patients (incidence is higher in ICU patients 50-60%). Magnesium level less than ~1.6mg/dL is defined as hypomagnesemia. Some epidemiological studies have reported that presence of hypomagnesemia is associated with increased morbidity and mortality. But due to many other confounding factors, direct connection is unclear.
Patients with mild hypomagnesemia are largely asymptomatic. Severe hypomagnesemia (<0.8mg/dl) can lead to cardiovascular, neuromuscular and CNS dysfunction (listed below). However since hypomagnesemia is almost always concurrent with hypocalcemia and hypokalemia it is difficult to attribute symptoms to hypomagnesemia itself. Note how many of the listed symptoms below are classically found with hypocalcemia and hypokalemia.
- Neuromuscular manifestations
- Positive Chvostek’s and Trousseau’s sign
- Muscle cramps
- Fasciculations and tremors
- Muscle weakness
- In vented patients, hypomagnesemia can be associated with delays in weaning due to respiratory muscle weakness.
- Neurologic manifestations
- Convulsions
- Athetoid movements
- Delirium
- Coma
- Cardiac manifestations
- Arrhythmias – torsades de pointes
- ST segment depression, flattened T waves, prolonged PR and QT/QTc intervals
- Enhanced sensitivity to digitalis toxicity
Our patient did not have any of these symptoms.
Take Home Messages
- Magnesium is an important cation for enzymatic reactions and physiologic processes in the human body.
- Magnesium homeostasis is maintained by the intestinal tract, bones and the kidneys.
- FeMg can be an important tool to differentiate between GI or kidney etiologies for hypomagnesemia. FeMg >15% in the setting of hypomagnesemia can suggest urinary magnesium wasting.
- Unlike other electrolytes, the majority (60-70%) of the filtered magnesium is reabsorbed at the thick ascending limb.
- Hypokalemia and hypocalcemia can be associated with hypomagnesemia.
- Cetuximab, an EGFR inhibitor, causes decreased expression of TRPM6 (Mg transporter in DCT) leading to urinary magnesium wasting.
- Chronic use of thiazides also causes urinary magnesium wasting by decreasing magnesium transport by decreased TRPM6 expression, independent of EGFR.


