 Hyponatremia can be broadly divided into two fundamental categories: “true hyponatremia” (in which the serum sodium concentration is truly less than normal), or “pseudohyponatremia,” in which the serum sodium concentration is actually normal but erroneously reported as low due to the presence of either hyperlipidemia or hyperproteinemia.
Hyponatremia can be broadly divided into two fundamental categories: “true hyponatremia” (in which the serum sodium concentration is truly less than normal), or “pseudohyponatremia,” in which the serum sodium concentration is actually normal but erroneously reported as low due to the presence of either hyperlipidemia or hyperproteinemia.
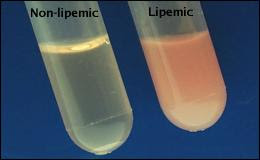
To understand why this is the case, it’s first necessary to understand that human plasma is normally composed of 93% plasma water and 7% proteins & lipids. Furthermore, it is necessary to understand that most clinical laboratories measure sodium using an indirect ion-selective electrode (ISE) which involves diluting the original blood sample in a 1:10 ratio and measuring whole plasma sodium based on the assumption that the sample is composed of 93% water. Thus, anything which increases the protein or lipid concentration of plasma will lead to an erroneously low sodium concentration. It occurs more often with hyperlipidemia than with hyperproteinemia, and one example would be in patients with familial hypercholesterolemia where their blood is highly lipemic.
One way to avoid measurements of pseudohyponatremia is to use a direct ion-sensitive electrode, which measures only the aqueous phase of an undiluted blood sample; however, in most labs this is not done routinely.
Pseudohyponatremia should also be differentiated from dilutional hyponatremia, in which an osmotic shift of water from cells to the vascular space following mannitol or IVIG infusions results in true (but hypertonic) hyponatremia.



Re: Ummat Syed – The laboratory measurement of sodium assumes plasma is 93 % water. In states of extreme lipemia or proteinemia the percentage of water may is not 93% thus erring the calculation due to higher volume of lipid/protein in the test sample.
could you explain further why measuring sodium in a 93% water assumption will enable pseudohyponatremia if lipids are in excess. Sorry don't get it.
Thankyou in advance,
Medical student