 The much awaited results from the ACCORD-BP trial were recently presented at the ACC scientific session in Atlanta and published online in the NEJM on March 14th. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) was a major initiative (estimated cost of $300 million) funded by the NIH to answer important questions about the “intensive” management of glycemic control (HgbA1c less than 6 vs. 7-7.9) blood pressure control (SBP less than 120 vs. less than 140) and dyslipidemia (statin + fibrate vs. statin alone) in high risk patients with type 2 diabetes. The NIH stopped the intensive blood sugar lowering strategy on February 6, 2008, due to safety concerns. The results were published in the NEJM on June 12, 2008 and actually showed an INCREASE in mortality and no decrease in cardiovascular events in the intensive glycemic arm (A1c less than 6) compared to the standard glycemic (A1c 7-7.9). The blood pressure and lipid treatment trials continued until the planned end of the study in June 2009. The results of were published online in the NEJM on March 11th. The BP arm study examined the well-established epidemiological data that lower BP is associated with lower cardiovascular events.
The much awaited results from the ACCORD-BP trial were recently presented at the ACC scientific session in Atlanta and published online in the NEJM on March 14th. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) was a major initiative (estimated cost of $300 million) funded by the NIH to answer important questions about the “intensive” management of glycemic control (HgbA1c less than 6 vs. 7-7.9) blood pressure control (SBP less than 120 vs. less than 140) and dyslipidemia (statin + fibrate vs. statin alone) in high risk patients with type 2 diabetes. The NIH stopped the intensive blood sugar lowering strategy on February 6, 2008, due to safety concerns. The results were published in the NEJM on June 12, 2008 and actually showed an INCREASE in mortality and no decrease in cardiovascular events in the intensive glycemic arm (A1c less than 6) compared to the standard glycemic (A1c 7-7.9). The blood pressure and lipid treatment trials continued until the planned end of the study in June 2009. The results of were published online in the NEJM on March 11th. The BP arm study examined the well-established epidemiological data that lower BP is associated with lower cardiovascular events.
Participants
• 4733 high-risk patients with DM2 (mean age, 62; 48% women)
• HgbA1c >7.5%
• >40 y/o with established CVD or
• >55 y/o with evidence of atherosclerosis, albuminuria, LVH or at least 2 additional risk factors for CVD (dyslipidemia, HTN, smoking or obesity).
• SBP between 130 -180 mmHg taking three or fewer meds for HTN
Exclusion criteria
• BMI >45, Creatinine >1.5 mg/dL, and other serious illness.
Study
• Patients were assigned to intensive BP control (target SBP less than 120) or standard BP control (target SBP less than 140)
• Mean follow-up was 4.7 years.
• Any FDA approved antihypertensive agent could be used to achieve targets
• Primary outcome was the first occurrence of a major cardiovascular event, which was defined as the composite of nonfatal MI, nonfatal stroke, or cardiovascular death.
Results
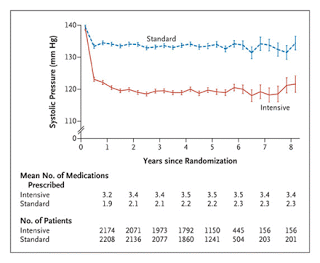
• Mean SBP and DBP at baseline were 139 and 76 mmHg in both groups.
• At 1 year, average SBP levels were 119 vs 134 mmHg (Intensive vs. Standard). This was achieved by prescribing more meds in the intensive group (at 1 yr 3.4 vs 2.1 meds).
• At 5 years, the rate of adverse cardiovascular events was 1.9% /yr vs. 2.1% /yr (HR, 0.88; P=0.2).
• Death rates were similar in the two groups.
• No secondary analysis was strongly positive, except that stroke incidence was significantly lower in the intensive-care group than in the standard-care group (0.32% vs. 0.53%).
• The intensive group had a higher rate of adverse events (3.3% vs. 1.3%), with more decrements in renal function and more episodes of syncope, bradycardia, hyperkalemia, and hypotension.
Several limitations of the ACCORD BP trial
1. Trial had an open-label design
2. The rate of cardiovascular events was lower than the expected rate in the standard group which reduced the statistical power calculation.
3. Patients 79 y/o were not included
4. SBP goal of less than 140 was used and not less than 130 which is currently recommended by JNC7

