 Parvovirus B19 (PVB19) is a small, non-enveloped, single-stranded DNA virus belonging to the Erythrovirus genus, named for their tropism for erythroid precursor cells. It is the only known parvovirus to infect humans. By late adulthood, most people have IgG Anti-PVB19 serology demonstrating previous exposure, often from an asymptomatic infection. However, infection with PVB19 may cause a variety of clinical syndromes including fifth disease (‘Erythema infectiosum‘, a childhood viral exanthem), a polyarthropathy, pure red cell aplasia and hydrops fetalis in utero. The nephrologist may encounter PVB19 in 3 broad settings:
Parvovirus B19 (PVB19) is a small, non-enveloped, single-stranded DNA virus belonging to the Erythrovirus genus, named for their tropism for erythroid precursor cells. It is the only known parvovirus to infect humans. By late adulthood, most people have IgG Anti-PVB19 serology demonstrating previous exposure, often from an asymptomatic infection. However, infection with PVB19 may cause a variety of clinical syndromes including fifth disease (‘Erythema infectiosum‘, a childhood viral exanthem), a polyarthropathy, pure red cell aplasia and hydrops fetalis in utero. The nephrologist may encounter PVB19 in 3 broad settings:
Post-Transplant
The incidence of PVB19 infection post kidney transplantation is hard to accurately determine but is likely in the range of 1-10%. Infection tends to occur in the first year and frequently in the first few weeks, suggesting possible donor transmission but the mechanism of infection/trans mission is unknown. Clinical syndromes are mainly acute anemia and chronic pure red cell aplasia, although a pancytopenia may develop. The few cases I have seen have been easy to identify, as patient presented with a profound isolated anemia. The diagnosis of chronic anemia or pancytopenia may be more protracted as these are obviously common post-transplant complications (graft dysfunction; drugs-immunosuppressants, anti-virals, co-trimoxazole, ACE inhibitor; other infections-CMV etc.). Treatment consists of reducing immunosuppression (usually the anti-metabolite) and IVIg. Similar to other viral infections (CMV, Polyoma viruses), there is some thought that PVB19 may be associated with allograft dysfunction or even acute rejection (?injured endothelium exposing previously hidden epitopes; no hard evidence for this however). A case series of thrombotic microangiopathy with PVB19 infection post transplant isolated PVB19 DNA from graft biopsies, however, overall the evidence for PVB19-induced allograft dysfunction is weak at present.
Glomerular Disease
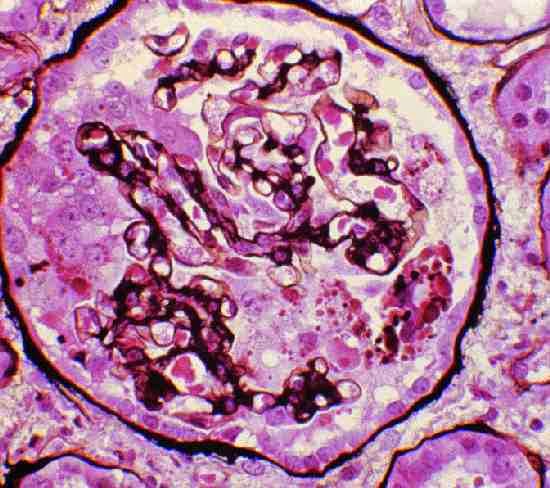
PVB19 may cause a variety of glomerular lesions in immunocompetent hosts. These were first noted as associations with PVB19 viremia, however, subsequently viral DNA has been identified in renal cells from biopsy tissue. The most well described pattern of injury is FSGS, particular a collapsing glomerulopathy (see image), where the PVB19 genome has been detected in podocytes and parietal epithelial cells by PCR. Proliferative glomerulonephritis has also been temporally associated with PVB19 infection. A syndrome similar to acute post-infectious glomerulonephritis may occur, displaying hypocomplementemia, endocapillary and mesangial proliferation with subendothelial electron dense deposits. Acute glomerulonephritis may be more common in patients with sickle cell disease after an aplastic crisis due to PVB19. Given the ubiquitous nature of PVB19, it is likely that host factors contribute to why certain individuals manifest glomerular disease, with genetic factors, as always, being implicated. Viral DNA may be found in renal tissue after the acute infection has passed and the glomerular lesions will not necessarily improve with resolution of the infection.
Dialysis Patients
Patient with ESRD are considered to be at risk for aplastic crisis due to PVB19, albeit with a paucity of data to support that claim. The presence of abundant immature erythroid cells (due to EPO use), a relative immunosuppressed state and some particular patient populations (e.g. sickle cell disease) lead to an increased theoretical risk of this complication.
PVB19 may be diagnosed using serology although immunosuppressed patients may not mount an adequate antibody response so PCR viral load is commonly employed. Treatment is non-specific and is generally supportive. IVIg is often used as these pooled preparations have anti-PVB19 antibodies, although randomized controlled data supporting its use is not available. Cutting back immunosuppression in transplant patients may also be of benefit, initially by reducing/stopping the anti-metabolite (logical as mycophenolate frequently induces bone marrow suppression independently). Also, tacrolimus is considered by some to be particularly conducive to PVB19 infection and a switch to cyclosporine may be a next step.
To summarize, PVB19 is important to the nephrologist from a clinical perspective particularly for its tendency to cause isolated severe anemia (or pancytopenia) in our immunosuppressed patients. It may also cause glomerular disease in immunocompetent patients and should be a differential diagnosis for otherwise unexplained glomerulopathy, especially collapsing FSGS. It also provides clues from a research perspective to mechanism of glomerular disease/sclerosis.

