Chronic kidney disease
(CKD) patients develop significant changes in bone mineral density (BMD) and
bone structure, which together with an increased risk for falls, put them at high
risk for fracture. This can be due, in part, to renal osteodystrophy, since bone
loss and reduced bone quality may be present in both
low- (osteomalacia and adynamic bone disease) and high-turnover bone
disease (osteitis fibrosa and mixed uremic osteodystrophy). In addition, some
patients may have underlying traditional risk factors for bone loss, such as
early menopause or smoking. The heterogeneity of bone loss in patients with CKD
complicates its management, requiring frequently a combination of
interventions. Performing a bone biopsy is the gold-standard to characterize
potential etiologies though not frequently available in most centers.
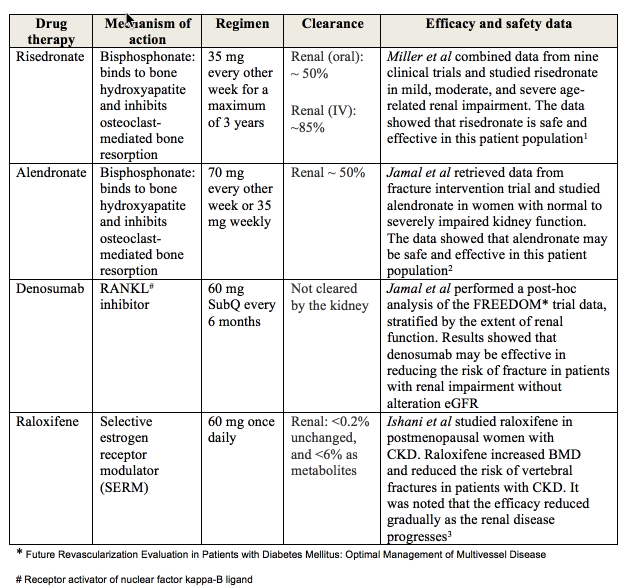
One of the major
challenges of treatment of osteoporosis in patients with eGFR below 30
mL/minute is the limited available data. Bisphosphonates are usually not
advocated in this population since most agents have renal clearance and
patients were frequently excluded from clinical trials. Post-hoc analyses of
studies in postmenopausal women with grades 4 and 5 CKD and a definite
diagnosis of osteoporosis revealed efficacy and short-term safety of
bisphosphonates, denosumab, and raloxifene in addition to calcium and vitamin D
supplementation. Bisphosphonates demonstrated increase in BMD, and reduction in
vertebral fracture incidence regardless of degree of renal dysfunction. To
date, anti-osteoporotic agents have not been recommended in CKD stage 5D due to
lack of data on security and on its beneficial impact against fracture. Though
some small studies have reported an amelioration of BMD with these drugs in
hemodialysis patients.
renal impairment are at higher risk for hypercalcemia due to calcium and
vitamin D supplements, or for hypocalcemia if taking denosumab therapy.
Therefore, serum calcium, phosphorus, parathyroid hormone, 25-hydroxyvitamin D
should be monitored at least every four months. Furthermore, renal function
should be routinely measured in patients taking bisphosphonates. Markers of
bone turnover, including but not limited to C terminal telopeptide (CTX), N
terminal telopeptide (NTX), and pyridinolines (PYR) are metabolized and/or
excreted renally, and will accumulate in renal dysfunction. Therefore, they
should not be used to monitor response to therapy in patients with eGFR below
30 mL/minute. Despite neither predicting fracture risk nor the type of renal
osteodysthrophy, bone densitometry of the hip and spine may be performed to
monitor for changes in BMD. In complex patients, a bone biopsy should be
considered prior to initiating osteoporosis treatment.
BMD (T-score below 2.5) associated with fragility fracture and grades 4 or 5
CKD, pharmacologic therapy might be considered after excluding all other
CKD-related low BMD diagnoses.
bisphophonates are typically recommended based on clinical experience and
existing data.
bisphosphonates should be used as a last resort only in patients who cannot
tolerate previous therapies and are at high risk for multiple fractures.
significant potential problem with IV bisphosphonates (Zoledronic acid). It is
dependent on both dose and infusion rates.
nephrotoxicity from IV bisphosphonates include acute tubular necrosis and
collapsing focal segmental glomerulosclerosis.
to guidelines for monitoring renal function prior to each dose and temporarily
withholding therapy in the setting of renal insufficiency, may help prevent
nephrotoxicity from these agents. Denosumab can be an interesting alternative
since it is not renally excreted. Further studies are required in patients with
CKD.
increase bone formation, such as teriparatide and anti-sclerostin monoclonal
antibodies (romosozumab and blosozumab) seem to be promising alternatives for
treating osteoporosis in CKD patients with low-turnover bone disease.
BCPS, FCCP