Acquired Cystic Kidney Disease in the ESKD population
Acquired cystic kidney disease (ACKD) was first described in 1847 by Simon while studying patients with glomerulonephritis (Bright’s Disease). However, it was not until 1977 when ACKD was “rediscovered” by Dunnill and his colleagues via autopsy studies of kidneys from dialysis patients. Now, we define ACKD in patients with kidney failure of any etiology by the finding of four or more cysts of varying sizes involving both kidneys with the absence of hereditary cystic disease. These patients will have either small or normal-sized kidneys as compared to the enlarged kidneys found in autosomal dominant polycystic kidney disease. ACKD tends to be more common in men and in the black population. There is an increased risk of developing ACKD with longer dialysis vintage, however no differences exist between the modalities of dialysis.
The precise pathogenesis of ACKD is not known. It is believed that the activation of proto-oncogenes and release of growth factors with resulting inflammation leads to tubular cell hyperplasia and cyst formation. This is accompanied by the loss of nephron mass and compensatory hypertrophy.
Most patients with ACKD are asymptomatic and the diagnosis is made incidentally via abdominal imaging ordered for other indications. The most common symptoms patients present with are hematuria, flank pain, and rarely urinary tract infection. Cyst rupture and hemorrhage can occur as there is a progressive increase in the number and size of cysts over time. Rarely, cyst hemorrhage is severe enough to extend into the perirenal area and cause significant hypotension. And finally, ACKD is occasionally found in the work-up of a patient with erythrocytosis or progressively decreasing erythrocyte stimulating agent requirements.
The Risk of Malignant Transformation
A major concern in patients with ACKD is the risk of malignant transformation into renal cell carcinoma (RCC). The incidence of RCC as a complication of ACKD is up to 7% over a 10-year period, with an almost 100-fold increase in risk compared to that of the general population. RCC in the setting of ACKD is more common in males, younger patients, and black as compared to white patients. Furthermore, there is a higher rate of multicentricity and bilaterality but a lesser degree of metastasis at the time of diagnosis when comparing to non-ACKD patients with RCC. The incidence of RCC in ACKD does increase with the duration of dialysis.
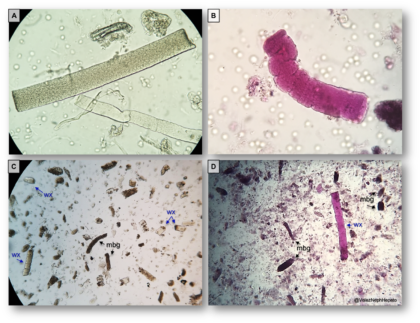
The common variants of RCC include clear-cell (conventional), papillary and chromophobe types. However, two more subtypes have been described specifically in association with ESKD as certain RCC forms showed characteristic features that could not fit into the current classification system. ACKD-associated RCC (ACKD-specific) and clear-cell-papillary RCC (not ACKD-specific) were first recognized in 2013 by the International Society of Urologic Pathology (ISUP) Vancouver Classification and accepted into the 2016 WHO classification of tumors. A gross specimen from a patient on dialysis with ACKD complicated by RCC is shown in Figure 1.
Figure 1. ACKD associated RCC on a gross specimen
With an increased risk of kidney malignancy in patients with ESKD, some authors have recommended screening patients on dialysis for ACKD and associated tumors. However, the target population, optimal screening modality, and the true benefits of screening have not been clearly elucidated. With a median 5-year survival of about 35% and the large burden of cardiovascular disease with resulting morbidity/mortality, the cost-effectiveness of routine screen remains debatable.
Sarasin and colleagues evaluated screening strategies in an effort to elaborate on potential benefits. They performed either CT or ultrasound imaging every three years on all dialysis patients and then annual imaging if cysts were found. In their study, they found that in patients with a life expectancy of 25 years, screening may decrease cancer deaths by half and offer as much as a 1.6-year gain in life expectancy. However, for the majority of patients, routine screening did not offer a significant benefit. Ishikawa and colleagues found that screening provided a survival benefit of 3.3 years in death from all causes after adjustment for age and dialysis vintage. As with the Sarasin study, young dialysis patients benefited the most. Furthermore, in this study, the survival rate for patients with RCC detected by screening was better than that in the group where RCC was detected by symptoms.
The European Renal Association and European Dialysis and Transplant Association suggest screening kidney transplant candidates for the presence of kidney cancer by ultrasound. The American Society of Transplantation recommends screening patients at high risk for renal cell carcinoma with both radiographic imaging and urine cytology. However, they do not define high risk and thus it is still left open to interpretation and largely nephrologist/institution dependent. In those who do perform screening, the common practice is to: ultrasound or CT image asymptomatic dialysis patients after 3 years of RRT and then every one to three years thereafter, with yearly screening in those with cysts, or more frequently depending on the cyst complexity.
A recent twitter survey by Dr. Roger Rodby (@NephRodby) showed that the majority of online respondents did not have a screening policy in place for their ESRD patients (Figure 2)
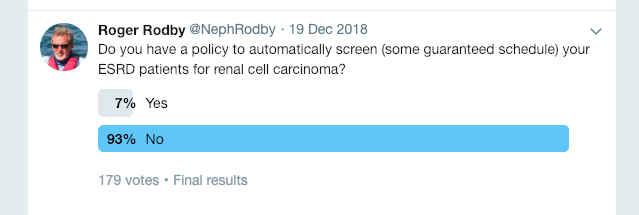
Figure 2: Twitter Poll by @NephRodby
What do we make of this data? We recently had a 58 year-old-male who was on HD for 15 years who died of metastatic RCC. Basing medical management decisions on anecdotal experiences is not a prudent policy. However, it is impossible not to wonder If we had an ACKD RCC screening policy, could this cancer have been detected in time to make a difference and perhaps even save his life? If such an RCC screening policy were to be developed, it should not include all patients with ESKD. It should be started after some defined time on dialysis (e.g. 5 years), in patients less than some defined age (e.g. 50 years), and with subsequent testing every few years thereafter. To screen or not to screen, that indeed is the question.
Post by: Cecille Marie C. Sales, MD (@SalesCecille)
1st Year Nephrology Fellow, Rush University Medical Center (@Rush_Nephrology)