In an ideal world, patients would present to our clinic with straightforward conditions, allowing us to confidently treat the patient with effective medications which will likely have much more benefit than harm. However, we also commonly encounter patients where the optimal approach to treatment is less clear, and IgA nephropathy can sometimes present this way.
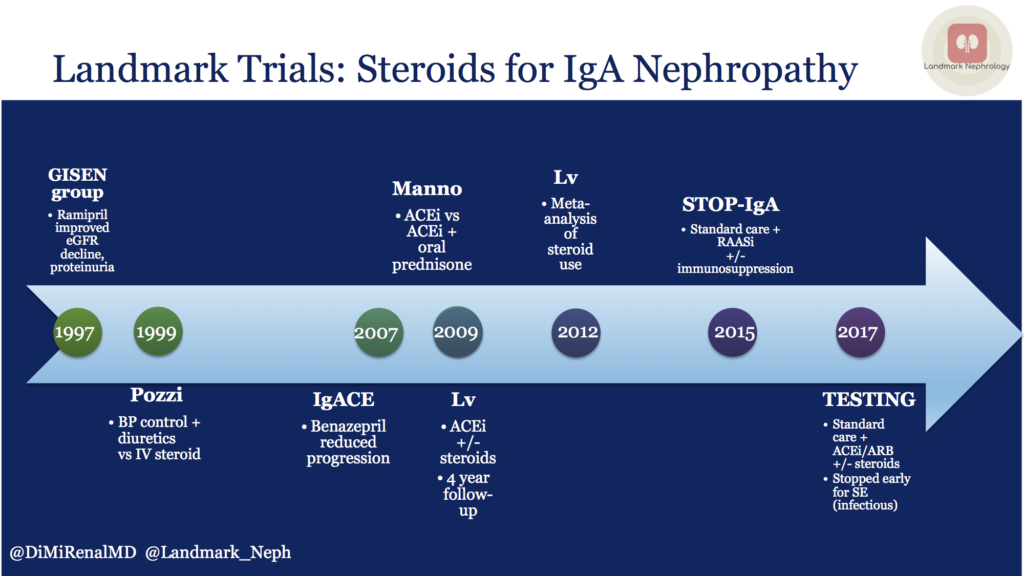
First, a word on renin-angiotensin-aldosterone system (RAAS) blockade as standard of care. We know that RAAS blockade helps to reduce proteinuria. The GISEN group in Italy showed that ramipril reduced proteinuria and rate of eGFR decline in non-diabetic patients with >3 grams per day of proteinuria. More specifically for IgA, the IgACE study in 2007 showed that in patients with biopsy-proven IgA nephropathy and moderate proteinuria, treatment of ACEi reduced progression of kidney damage.
Thus, RAAS blockade remains standard of care for proteinuric kidney disease, and this remains true in IgA nephropathy.
It is useful to review the Landmark Trials in IgA Nephropathy so that we know what we know (and what questions remain) when treating patients with biopsy-proven IgA who have relatively preserved kidney function and some degree of proteinuria. We are not talking about the patient whose only clinical manifestation is microscopic hematuria, nor is this relevant to the patient with rapidly-progressive glomerulonephritis.
One of the first Landmark trials to evaluate steroids for IgA nephropathy was published by Pozzi in 1999. This study randomized 86 patients. Investigators compared supportive therapy with blood pressure control and diuretics to IV methylprednisolone. RAAS blockade was not standard of care at that time, so patients may or may not have received an angiotensin converting enzume (ACE) inhibitor or angiotensin receptor blocker (ARB). This study showed that 6 months of IV steroids reduced proteinuria and protected against deterioration in kidney function, WITHOUT adverse events during follow-up, including specifically infectious serious adverse events.
A decade later, Manno and colleagues randomized 97 patients to ACE inhibition alone versus ACE inhibition plus oral prednisone. Follow up time was relatively long at 8 years, and combination therapy protected against progression of kidney disease. Again, there were no major adverse events, including serious infections.
In the same year, Lv and colleagues published results from a randomized control trial (63 patients) comparing ACE inhibition with or without oral prednisone, with 4 year follow-up, and found similar results. Namely, combination therapy protected against progression of kidney disease, without major adverse events. Based on these studies, steroids were looking pretty good for treatment of this subgroup of patients.
In 2012, the same lead author published a meta-analysis of trials from 1966 to 2011. It was noted that steroid therapy was associated with less kidney dysfunction and proteinuria, but also with a 55% higher risk of serious adverse effects, though NOT severe infections. Most studies used high dose, short term steroids and the data was limited. Additionally, ACEi/ARB was not mandated in most studies.
The STOP-IgA randomized trial (337 patients) in 2015 evaluated supportive care, which included RAAS blockade, with supportive care plus immunosuppression (either steroids for preserved kidney function or cyclophosphamide, azathioprine, and prednisone for eGFR between 30-59.)
Interestingly, this study showed that immunosuppressive therapy did NOT improve outcomes, and was associated with more adverse effects.
Most recently, the TESTING randomized trial (262 patients) set out to find answers, given the conflicting data from previous studies. All patients received blood pressure control including RAAS blockade and were randomized to placebo versus oral steroids. Unfortunately, recruitment was discontinued early due to an excess of serious adverse events, mostly serious infections. The number of primary outcomes are small, but the data do indicate better primary kidney outcomes with steroids compared to placebo.
Like most treatments that we provide to our patients, we must try to weigh the risks and benefits in each individual patient, and further studies are needed to help to guide our clinical decision-making.
Post by: Diana Mina, MD
Nephrologist, Dallas Renal Group
Landmark Nephrology is an online learning tool designed to collect landmark trials in nephrology and distribute content that makes learning nephrology fun and easy.
Please visit us to check out our topic-specific content including videos, visual abstracts, quizzes, and a new slide-share portal to facilitate the exchange of educational material within the nephrology community.
We love collaboration so contact us as landmarknephrology@gmail.com or find us on Twitter to get involved!


