Scientific technology is advancing quicker than ever before. Something I never thought I would be saying in my lifetime is that science now has the technology to create an Organ-On-A-Chip. An Organ-On-A-Chips is an engineered in vitro modelled organ-like culture. They contain organ specific cells arranged on different scaffolds that allow for mimicking of physiology and potentially pathophysiology of organ systems. They provide the possibility of reducing the need for research animals as well as strengthen and advance the connection between basic cellular pathways and drug development research. Organ-On-A-Chip’s are a human cell culture chip that can simulate detailed molecular pathways, signaling, and whole organ/organ system physiological responses.
Cell cultures have been an important part of medicine since the 1950’s. The first human cells successfully cloned; HeLa cells, came from a deceased patient without consent (Henrietta Lacks) with cancer and have since been utilized in research ranging from the first polio vaccine to gene mapping and tumor xenografts. Cell cultures, in general, are a mainstay application in molecular biology laboratory for examining the normal and abnormal biochemistry of cells. However, cell culture continues to entrenched in a fair amount of controversy in the biomedical field. For instance, do cultured cells bear any resemblance to their derived in situ relative? Current cell culture techniques present many disadvantages. For example, many cell types are of non-human origin, immortalized, of a single cell type, grown in static conditions, on a plastic surface, and in a 2D format that is quite different from the dynamic internal milieu this same cell will experience in the body. Current 2D cell culture work oversimplifies the cell’s microenvironment and extracellular matrix, and you could imagine if you are testing the toxicity of a novel drug compound, the results may differ significantly between 2D culture and in vivo experiments. Animals, predominantly mice and rats these days, are used as models to study physiological and pathophysiological states in vivo, however, they also possess some big downsides. Species-specific differences are evident in all organ systems, leading many to question how well this information can be translated into understanding human physiology. Thus, developing a tool, that mimics human organ systems, but doesn’t limit a study to a single cell type, oversimplification of a complex system, or specifies specific boundaries, provides a rigorous and reproducible key for drug development. Organ-On-A-Chips can efficiently and effectively predict potential drug toxicity and disposition, especially in the kidney, which is often a site of toxicity during drug development. According to a research study by MIT, only about 14% of clinical trials drug candidates will hit the market. Organs-on-a-chip could help significantly increase these outcomes.
In drug development research, cell culture models fail to accurately predict toxicity and animal studies are challenging, making akidney chip a possible solution. These organ chips can even be broken down into specific parts of that organ. For example, something a basic cell culture could not achieve, the nephron chip can contain multiple cell types from all nephron segments and interstitium to demonstrate urea concentration in the loop of Henle, reabsorption in the proximal tubule, and function glomerulus filtration. There is even a proximal tubule chip, mimicking a major site in the nephron responsible for the clearance of a drug as well as the principal target for nephrotoxicity induced by a drug. Kyung-Jin Jang et. Al. were the first to use the proximal tubule chip, which consists of primary human kidney proximal tubular epithelial cells to examine mechanisms of drug delivery and possible toxicities. What they discovered was that this chip accurately displayed the morphology of the proximal tubule, its full function, and accurate drug toxicity responses when it was exposed to apical fluid shear stress. Overall, they supported the use of this chip as an accurate and efficient method to study human-relevant renal toxicity in preclinical studies.
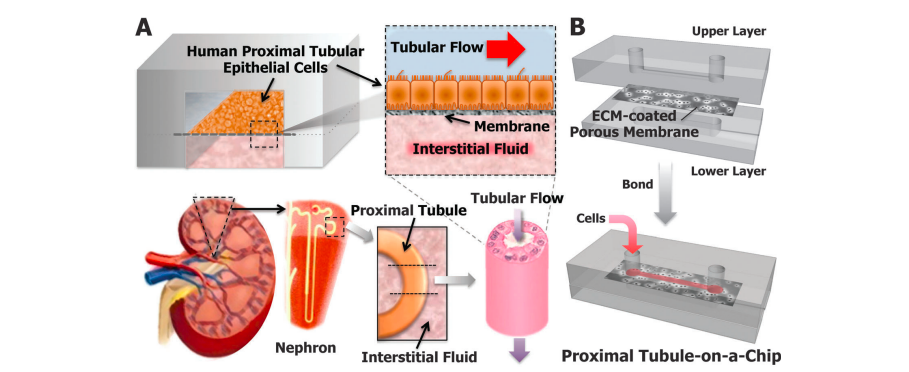
More recently, studies began to expand into studying microphysiologic systems (MPSs), a field that encompasses Organ-On-A-Chips. The following research is part of an initiative (publicly funded) whose goal it is to drive the research of human cell-derived models in order to advance the drug development field. One recent development was performed by Weber et al., who developed a rather interesting and novel proximal tubular MPS. It exhibits long-term viability, a previous limiting factor mentioned above, and retains function and polarized expression of proteins essential for reabsorptive and secretory transport. It also efficiently performs perilous biochemical synthetic activities and responds to physiological stimuli. Among all the testing this model went through, it was able to transport organic anionic solutes across the epithelial cell, was sensitive to selective inhibitors, and responded to nephrotoxins displaying secretion/expression markers of tubular cell injury. The MPS, when compared to conventional screening methods, holds the potential to better predict renal outcomes in drug development.
The idea of Organ-On-A-Chips has even made its way up to the International Space Station to provide scientific data for future human spaceflight and diseases here on earth. This mission, “Tissue Chips in Space Project” includes support and guidance by the National Institute of Health’s National Center for Advancing Translational Sciences (NCATS) as well as the International Space Station U.S. National Lab. Four different types of chips will make their way up to space, including: cartilage, bone and synovium (joint tissue) chip developed by MIT, a lung host defense chip developed by the Children’s Hospital of Philadelphia, a blood brain barrier chip developed by biotech specialists, and finally a kidney chip developed by the University of Washington. Because it takes so long for conditions to develop, these chips are being sent to a place where time speeds up, space. Scientists will finally be able to observe things such as the early stages of kidney disease, conditions such as osteoporosis, and kidney stones. These conditions can take years to form on earth, however, they develop in weeks or months in microgravity.
The development of these models provides the potential to observe multiorgan interactions, basic structures, and functions in a more accurate model. It also provides a prevailing screening tool for human disease as well as for testing novel treatments. Maybe by the time I finish my PhD, we will see more organs on chips and possibly an increase in some clinical trials successfully utilizing these chips.
Kasey Belanger
@BelangerKasey
PhD Student at Augusta University


