Introduction
Patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) are frequently on anticoagulation (AC) due to their increased risk of stroke and thromboembolism, particularly from atrial fibrillation. Management of AC in kidney patients undergoing invasive procedures can be a daunting task given that CKD and ESKD patients have an overall greater risk of both thromboembolic events and clinically significant bleeding compared with the general population. The recent surge in the use of novel oral anticoagulants (NOACs) adds an additional layer of complexity, especially since the periprocedural management of NOACs in CKD and ESKD patients has not been well-studied.
Individual hospitals and clinics often have their own established AC protocols, and adhering to local practice with regard to periprocedural AC management is recommended. The goal of this article is to provide a case-based overview of commonly prescribed AC and offer a framework for approaching CKD and ESKD patients taking these medications who are undergoing common nephrology-related procedures.
Case 1
A 76-year-old Caucasian man with a past medical history of atrial fibrillation, coronary artery disease, osteoarthritis, chronic kidney disease with baseline GFR of 54 mL/min, diet-controlled diabetes mellitus with fasting glucose in the normal range, and well-controlled hypertension is scheduled for kidney biopsy due to acute onset of nephrotic-range proteinuria and decline in GFR to 32 mL/min without a clear explanation. His current medications include carvedilol 25 mg twice daily, aspirin 81 mg daily, chlorthalidone 25 mg daily, and dabigatran 150 mg twice daily. You discuss the risks of the biopsy and advise that he should:
- Stop aspirin 7 days prior and stop the dabigatran one day prior to the biopsy.
- Stop aspirin 7 days prior and stop the dabigatran 3 days prior to the biopsy. Plan to admit him to the hospital for heparin bridging.
- Stop aspirin 7 days prior and stop the dabigatran 4 days prior to the scheduled outpatient biopsy. He does not require hospital admission for heparin bridging.
- Stop the aspirin 7 days prior to the biopsy and continue the dabigatran at a lower dose of 75 mg twice a day.
Answer: Choice C.
Explanation: This patient has a CHADS2-VASc score of 3 (for hypertension and age) and is at risk for periprocedural thromboembolism, but he does not have a prior risk of stroke or venous thromboembolism. On the other hand, bleeding is a common complication of kidney biopsy. The half-life of dabigatran in patients with moderately impaired renal function (GFR 30-50 mL/min) is around 16-18 hours. Stopping dabigatran 4-5 days prior to high bleeding risk surgery provides adequate time for drug clearance. Of note, dabigatran is 80% excreted by the kidney and 38% protein-bound, and it is the only dialyzable NOAC in the event of a major bleeding emergency. Doses of 150 mg twice daily are appropriate for patients with GFR ranging from 30-50 mL/min, but the dose should be reduced to 75 mg twice daily for patients with creatinine clearance ranging from 15-30 mL/min. Dabigatran should be avoided in dialysis-dependent patients with end-stage kidney disease.
In this patient’s case, simply reducing the dose would not decrease the risk of bleeding complications. Given dabigatran’s quick onset of action once resumed and the absence of prior history of thromboembolism in this patient, heparin bridging is unnecessary.
Case 2
A 48-year-old African American woman with a past medical history of hypertensive renal disease on hemodialysis via a right upper arm arteriovenous graft (AVG), cerebrovascular accident with residual right hemiparesis, and left lower extremity deep vein thrombosis diagnosed two months ago has been taking apixaban 2.5 mg twice a day. She has no history of clinically significant bleeding. The nurse at her outpatient dialysis unit was unable to appreciate a bruit or thrill in her graft this morning, and she has been sent to interventional nephrology for percutaneous thromboembolectomy (“declot”). You are concerned about possible bleeding risk because tissue plasminogen activator (tPA) and heparin are commonly given during this procedure. However, the patient’s potassium is 5.9 and she is 4 kg over her estimated dry weight. You decide to:
- Check the patient’s international normalized ratio (INR). If it is less than 3.0, go ahead with the declot, but advise using half the usual amount of heparin and tPA during the procedure.
- If there is no evidence of clinically significant bleeding, proceed with the declot and plan to dialyze the patient immediately post-procedure.
- Postpone the declot for one week and hold the apixaban, admit the patient to the hospital for intravenous heparin bridging, and place a temporary dialysis catheter in the right internal jugular vein.
- Hold the apixaban and schedule for outpatient declot in three days.
Answer: Choice B.
Explanation: There is a paucity of published literature on the safety of percutaneous AVG thromboembolectomy using tPA and heparin while on NOACs. INR does not monitor the activity of apixaban and would not be helpful in this patient. Postponing the declot for one week, initiating heparin bridge, and placing a temporary dialysis catheter would pose other risks to the patient and, in a patient with no major bleeding history, would likely be excessive. Holding the apixaban for a few days and rescheduling the procedure would be a reasonable option, except the patient is hyperkalemic and volume overloaded and in imminent need of dialysis. Data from the stroke literature suggests that, barring other contraindications to giving tPA, the risk of clinically significant bleeding with tPA administration in a patient taking apixaban is minimal. It is our clinical practice to proceed with an AVG declot on patients taking warfarin if INR is in the therapeutic range for its indication or on patients taking NOACs in whom there is no evidence of clinically significant bleeding. The lowest effective dose of tPA and heparin should be given: generally no more than 2,000 units of systemic heparin and 2-4 mg of tPA and 2,000-3,000 units of heparin administered directly into the AVG. If there is still concern about using tPA and heparin (“lyse and wait” method), declot may be performed with the aid of a mechanical thrombolytic device. The latter method offers similar results compared with “lyse and wait”; however, it is more expensive and requires the administration of intragraft heparin (but avoids the use of tPA).
Case 3
A 55-year-old Caucasian woman with a past medical history of atrial fibrillation on rivaroxaban, cerebrovascular accident four years ago, hypertension, insulin-dependent diabetes, morbid obesity, non-alcoholic fatty liver disease, and advanced chronic kidney disease (GFR ranging from 12-17 mL/min over the last 6 months) is being seen in the nephrology clinic for the first time for rising serum creatinine. She has been reluctant to see a nephrologist in the past but finally agreed to the referral due to persistent nausea, decreased appetite, and increased “forgetfulness.” She also reports worsening ankle swelling and shortness of breath, and she does not urinate as much as she used to despite doubling her dose of furosemide from 40 mg to 80 mg twice daily and adding metolazone to her diuretic regimen. Her oxygen saturation is 88% on room air but improves to 93-94% with supplemental oxygen at 2 liters per minute. She is unable to provide a urine specimen. Blood chemistries are notable for a potassium of 5.8 mg/dL (non-hemolyzed), serum creatinine of 8.7 mg/dL (GFR 6 mL/min), platelet count of 47,000 per microliter, and INR of 1.6. She agrees to hospital admission for initiation of hemodialysis once she has a central venous catheter placed. You call the inpatient nephrology service and recommend:
- Immediate temporary hemodialysis catheter placement after administration of fresh frozen plasma, stopping rivaroxaban for 4 days, heparin bridging, and deferred tunneled dialysis catheter placement until INR is less than 1.5 and platelet count is higher than 50,000 per microliter.
- Urgent treatment with recombinant coagulation factor Xa and tunneled dialysis catheter placement followed by same-day hemodialysis.
- Administration of prothrombin complex concentrate followed by temporary dialysis catheter insertion and tunneled dialysis catheter placement the next day.
- Immediate tunneled dialysis catheter placement followed by initiation of hemodialysis. Consider changing anticoagulation to warfarin.
Answer: Choice D.
Explanation: Rivaroxaban is a direct oral anticoagulant and its effectiveness is not monitored with INR. Significant bleeding complications following tunneled dialysis catheter placement, even for patients with coagulopathies or on anticoagulation and antiplatelet medications, are rare. Reversing anticoagulation in this patient is therefore unnecessary. In a severely coagulopathic patient requiring AC reversal, fresh frozen plasma (or prothrombin complex concentrate if urgently needed) can be used to reverse the AC effects of warfarin. Recombinant coagulation factor Xa can be used to reverse rivaroxaban and apixaban if needed.
Rivaroxaban is a Factor Xa inhibitor that has been increasingly used for atrial fibrillation in patients with advanced CKD and ESKD, but literature regarding its safety and appropriate dosing in ESKD populations is scarce and the rate of reported bleeding complications is high. Considering a change to warfarin may be appropriate given this patient’s progression to ESKD. Per 2014 American Heart Association guidelines, warfarin is the AC of choice for atrial fibrillation in patients with advanced CKD or ESKD.
Case 4
A 42-year-old Hispanic man with a past medical history of hypertension, heart failure with reduced left ventricular ejection fraction (30-35%), and mechanical mitral valve on warfarin. His INR has been within goal range of 2.5-3.5 over the last six months. He has had progressive chronic kidney disease that has now reached end-stage. He wants to start peritoneal dialysis so he can continue to work and travel. During surgical evaluation, he was noted to have a large inguinal hernia that needs to be repaired. He is concerned about stopping his anticoagulation for surgery. You inform him that:
- He has a high risk of perioperative thromboembolism due to his mechanical heart valve. Although both peritoneal dialysis catheter placement and inguinal hernia repair are generally low bleeding risk procedures, his risk of procedure-related bleeding is higher with AC use. He will need to stop his warfarin at least 5 days prior to the procedure and be bridged with unfractionated heparin or low-molecular-weight heparin.
- He has a high risk of perioperative thromboembolism due to his mechanical heart valve, and peritoneal dialysis catheter placement is a high bleeding risk procedure, although inguinal hernia repair is a low bleeding risk. He should reduce his warfarin dose to meet a new target INR of 1.5-2.0.
- He has a low risk of perioperative thromboembolism due to his mechanical heart valve, and both peritoneal dialysis catheter placement and inguinal hernia repair are high bleeding risk procedures. He should stop warfarin immediately and start a prophylactic dose of low molecular weight heparin in anticipation of surgery.
- He has a low risk of perioperative thromboembolism due to his mechanical heart valve, and both peritoneal dialysis catheter placement and inguinal hernia repair are low bleeding risk procedures. He should maintain warfarin at current dose with goal INR of 2.5-3.5 perioperatively.
Answer: Choice A.
Explanation: Because of his mechanical mitral valve, this patient has a high risk of perioperative thromboembolism if he stops anticoagulation prior to surgery. Bleeding risk is higher for patients on AC undergoing hernia repair. Bleeding complications associated with peritoneal dialysis catheter placement are rare, but are more likely to occur with AC use. The American College of Cardiology and American Heart Association recommend heparin bridging with either unfractionated heparin or low-molecular-weight heparin once the INR falls below the therapeutic range (INR < 2.5 in our patient).
Pre-Procedural Risk Assessment
Not all CKD and ESKD patients on AC are at equal risk of developing periprocedural thromboembolic complications, and not all procedures pose the same risk of bleeding. Understanding why the patient is on AC allows for appropriate stratification of thromboembolic risk (Table 1).
With the exception of kidney biopsy, most nephrology-related procedures (including endovascular procedures) have a low risk of associated bleeding, and frequently do not require an interruption of AC therapy or heparin bridging (Table 2). However, as in the case of the patient undergoing hernia repair and peritoneal dialysis catheter placement, AC itself may increase the risk of perioperative bleeding complications. Stopping AC and using periprocedural bridging heparin may be appropriate in certain situations to minimize the individual patient’s bleeding and thromboembolic risk simultaneously.
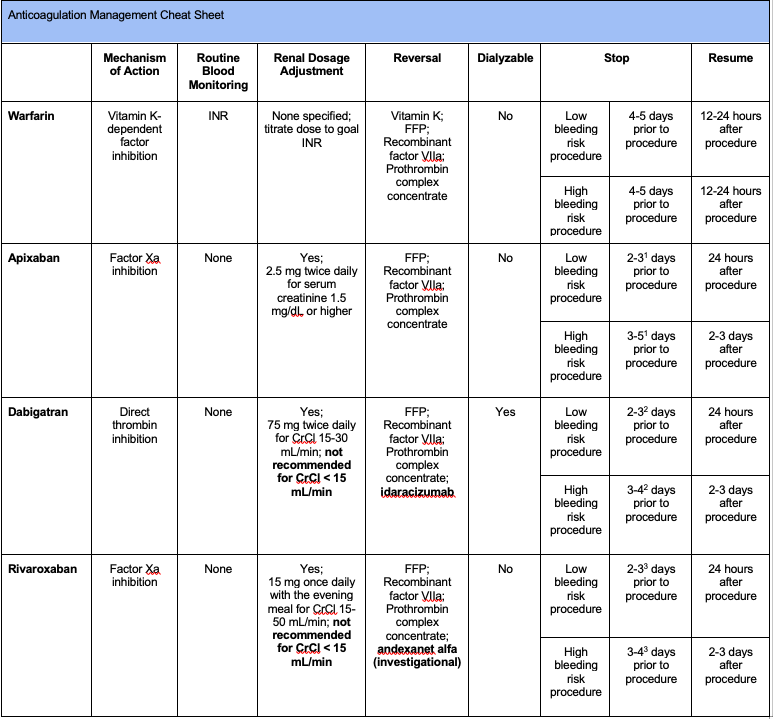
1Stop apixaban 2 days prior to a low bleeding risk procedure and 3 days prior for a high bleeding risk procedure if CrCl > 50 mL/min. Stop apixaban 3 days prior to a low bleeding risk procedure and 4 days prior for a high bleeding risk procedure if CrCl is 30-50 mL/min. Guidelines for stopping apixaban prior to procedure in patients with ESKD are not clearly established. It is reasonable to treat patients
2Stop dabigatran 2 days prior to a low bleeding risk procedure and 3 days prior for a high bleeding risk procedure if CrCl > 50 mL/min . Stop dabigatran 3 days prior to a low bleeding risk procedure and 4-5 days prior for a high bleeding risk procedure if CrCl is 30-50 mL/min.
3Stop rivaroxaban 2 days prior to a low bleeding risk procedure and 3 days prior for a high bleeding risk procedure if CrCl is 30 mL/min or higher. Stop rivaroxaban 3 days prior to a low bleeding risk procedure and 4 days prior for a high bleeding risk procedure if CrCl 15-29 mL/min.
Conclusion
Patients with CKD and ESKD have a paradoxical increased risk of bleeding and clotting. Periprocedural management of AC remains challenging in patients with kidney disease. It is important to (1) know why the patient is on anticoagulation to assess thromboembolism risk, (2) determine whether the proposed procedure or surgery has a generally low or high risk of bleeding, (3) understand the duration of action of warfarin and the NOACs to stop and resume AC at the appropriate time, and (4) determine if there is a need for heparin bridging.
Kidney biopsy has a high bleeding risk, but the risk of bleeding with endovascular procedures and tunneled dialysis catheter placement remains low, even when patients are on AC. Careful assessment of the patient’s individual risks is crucial to minimize periprocedural bleeding and clotting risks in patients with kidney disease.
Additional Resources:
Heparin bridging
Crystal Farrington, MD
ASDIN Fellow
Acknowledgments
This post is part of a collaboration between the Renal Fellow Network and the American Society of Diagnostic and Interventional Nephrology (ASDIN), whose mission is to provide excellence in dialysis access care to improve outcomes for patients with kidney disease. Special thanks to Aisha Shaikh, Edgar Lerma, and the ASDIN Education Committee. For more information about the ASDIN mission or membership, click here.



Realy informative
It is also important to clarify with patients when stopping or holding aspirin if it is being used for primary prevention or secondary prevention. The benefits may outweigh the risks of continuing aspirin therapy if secondary prevention with PCI even of > 12 months out from placement.