Introduction
Atheroembolic kidney disease develops due to occlusion of small renal arteries by cholesterol crystal emboli originating from the rupture of atheromatous aortic plaques. It is part of a systemic disease and embolization often affects other organs such as skin, gastrointestinal system, and brain. Kidneys are the most frequent target organ involved because of the proximity of renal arteries to the abdominal aorta and due to the presence of high renal blood flow.
Risk factors
Atheroembolic kidney disease is commonly observed in adults >60 years of age with widespread atherosclerosis. Other risk factors include male gender, diabetes, hypertension, hyperlipidemia, and smoking.
Precipitating event
Although atheromatous material can be dislodged spontaneously, it is frequently an iatrogenic disease and may follow endovascular procedures and vascular surgery including coronary bypass grafting and long-term anticoagulant or thrombolytic therapy.
Incidence
There is uncertainty regarding the exact prevalence of atheroembolic kidney disease probably due to sampling bias. In unselected autopsy series, the frequency is generally low, ranging from 0.8 to 4.4%. However, autopsy studies done in elderly patients and those who died after aortic surgery or aortography have reported an increased frequency from 12% to 77%.
Presentation
Manifestations vary from acute, sub-acute and chronic renal failure. Subacute presentation is the most common form and acute renal failure affects 20-30% of the patients.
Clinical outcome
The reported mortality varies from 64 to 81%. Around 33-61% of the patients require dialysis, of whom, 21-39% recover kidney function. Pre-existing chronic kidney disease and longstanding hypertension are predictors of poor outcome.
Histologic features
The classic lesion in atheroembolic kidney disease is the occlusion of arcuate and interlobular arteries with cholesterol crystal emboli. Rarely, small crystals can lodge in the afferent arterioles and glomerular capillaries as well. The crystals are recognized by the characteristic biconvex, needle-shaped empty clefts, appearing as “ghosts” because they are dissolved during routine histologic processing. The involvement is usually patchy, hence, careful examination of multiple step sections is necessary.
In the initial stages, the crystals are surrounded by red blood cells, fibrin, neutrophils, and eosinophils. Subsequently, mononuclear cells accumulate in the infiltrate. Multinucleated giant cell reaction surrounding the clefts is often present.
Over a period of time, the affected blood vessels get occluded by prominent intimal fibrosis. Ischemia secondary to vascular occlusion can lead to global or segmental glomerulosclerosis.
Other changes may include acute tubular injury, interstitial fibrosis, and tubular atrophy.
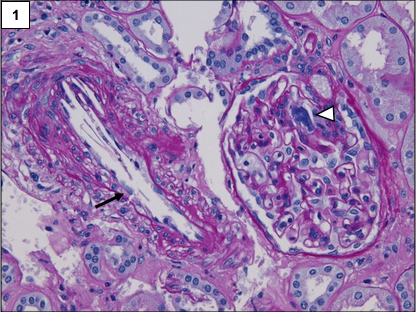
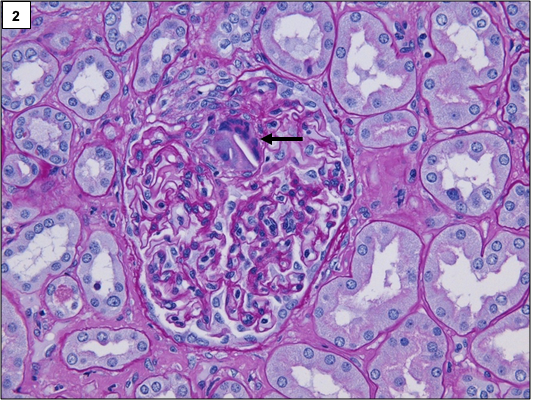
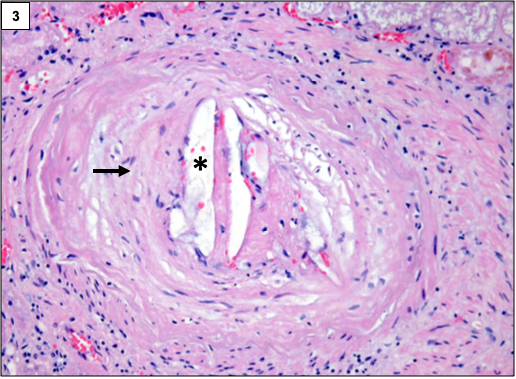
Differential diagnosis:
- Arteriosclerosis: Severe intimal fibrosis may mimic chronic forms of atheroembolic renal disease, but, there are no cholesterol clefts.
- Artifact: Artifacts due to compression by forceps or tissue processing may have slit-like lumina inside arterioles and small arteries that mimic cholesterol clefts.
- Vasculitis: There is multi system involvement similar to atheroembolic renal disease. The vessels show transmural inflammatory infiltrate without any cholesterol clefts.
Preethi Sekar, MD
Assistant Professor of Pathology,
KMCH Institute of Health Sciences & Research, Coimbatore, India.
[Picture courtesy-University of Chicago]

