Our endeavor for the next 2 months will be a focus on Lupus nephritis. This month we will cover general aspects of disease, introduction to subclasses and activity indexes.
Background and Demographics
- Systemic lupus erythematosus (SLE) is a systemic autoimmune and chronic disease
- It is more prevalent in woman than man across all age groups and populations; women to men ratio is highest at productive age, ranging between 8:1 and 15:1 and lower in prepubertal children at about 4:3
- The prevalence of SLE and the chances of developing lupus nephritis (LN) vary significantly between different areas of the globe and different races and ethnicities
- In the United States, the higher frequency of LN in African-American populations persists after adjustment for socioeconomic factors. The prevalence in U.S. is ranging widely between 4.8 to 87.5 per 100,000. This difference in prevalence of the disease can be explained perhaps by genetic predisposition as some “high-risk” genotypes and autoantibodies are more frequent in black patients
- African Americans and Hispanic SLE patients develop LN earlier, and have worse outcomes than white patients with SLE, including death and end-stage kidney disease.
- Patients with LN have a higher standardized mortality ratio (6–6.8 versus 2.4) and die earlier than SLE patients without LN.
- 10-year survival improves from 46% to 95% if disease remission can be achieved.
Pathogenesis
Multiple pathologic triggers and mechanisms are able of causing glomerular injury in SLE, and this triggers heterogeneity is reflected in the variable patterns of injury seen on renal biopsy and the different clinical presentations with lupus nephritis. There are at least two accepted hypothesis regarding the pathogenesis of LN:
- Circulating immune-complex disease: it is supported by serologic studies that showing high levels of circulating immune complexes, double-stranded DNA, other nuclear antigens and consumption of classic complement components (C1, C4, and C3). Serologic findings associated with circulating immune complexes and activation of complement correlate best with large subendothelial deposits seen by pathologic microscopy examination in class III/IV LN.
- In situ immune-complex disease: This theory implies specifically to membranous lupus nephritis as the result of the in situ reaction of unbound antibody with antigen.
Clinical presentation and laboratory testing
- The clinical manifestations of LN are often subtle and most commonly will be discovered by examination of the urine as opposed to physical examination.
- SLE patient should be evaluated at initial diagnosis and annually for renal involvement, even if they don’t have any signs and symptoms of renal disease. The evaluation is basically lab tests included renal function and urine analysis
- If there is a clinical suspicion of renal involvement, a renal biopsy is highly recommend step because early diagnosis and treatment of LN have a good prognosis
- However; there is no standardized guideline or indications when to do renal biopsy in SLE. In general, proteinuria more than 500 mg/ day or any level of proteinuria or hematuria with impaired kidney function that cannot be attributed to another cause, these findings might prompt the clinician to perform renal biopsy
- The disease could have subclinical course “Silent lupus”, even with class III/IV.
- There are other mechanisms that result in renal injury rather than immune-complex mechanism which can only be diagnosed with a biopsy, and require a different management like, thrombotic microangiopathy and lupus podocytopathy
- Other laboratory tests that should be considered that might correlate with disease activity: Anti-nuclear antibody (ANA), anti-double stands DNA antibody (anti-dsDNA), anti-cardiolipin antibody, anti-phosphlipid antibody, and serum complements level.
Grading of Lupus Nephritis
The most recent acceptable classification of lupus nephritis, published simultaneously in 2004 and modified in 2018 in kidney international and the journal of American Society of nephrology and it is called the “Modified International Society of Nephrology/Renal Pathology Society ISN/RPS Classification of Lupus Glomerulonephritis (2004,2018) (Table 1). The classification provides a standardized definition of each class, eliminating ambiguous findings and improving interobser agreement among renal pathologists. Of note, the classification is based on light microscopy and immunofluorescence findings because most of centers outside the United States might have no electron microscopy. Also it is recommended to provide the severity and the activity of the disease to provide prognostic information, and to guide the clinicians to better approach for treatment (Table-2).
Table 1 . Modified ISN/RPS Classification of Lupus Glomerulonephritis
| Class | Name | Definition | Comments |
| I | Minimal mesangial lupus nephritis | Normal by LM with mesangial deposits by IF or EM | May have other features such as podocytopathy or tubulointerstitial disease (beware of unsampled class III) |
| II | Mesangial proliferative lupus nephritis | Purely mesangial hypercellularity by LM with mesangial deposits by IF; may be rare subepithelial or subendothelial deposits by IF or EM (not by LM) | May have other features such as podocytopathy, tubulointerstitial disease (beware of unsampled class III), or thrombotic microangiopathy |
| III | Focal lupus nephritis | Active or inactive segmental or global endocapillary &/or extracapillary glomerulonephritis by LM in < 50% of glomeruli; usually with subendothelial deposits | Active (A) & chronic (C) lesions defined in Modified NIH Activity & Chronicity Scoring System; replaces A & C designation |
| IV | Diffuse lupus nephritis | Active or inactive endocapillary &/or extracapillary glomerulonephritis by LM in ≥ 50% of glomeruli | Omitted segmental (S) & global (G) designations due to lack of reproducibility & clinical correlation; A & C lesions as defined in Modified NIH Activity & Chronicity Scoring System |
| V | Membranous lupus nephritis | Global or segmental granular subepithelial deposits along GBM by LM & IF or EM; if class III or IV present, need to be in > 50% of capillaries of > 50% of glomeruli; ± mesangial alterations | May occur with class III or IV, which are designated class III/V or class IV/V, respectively |
| VI | Advanced sclerosing lupus nephritis | ≥ 90% of glomerular sclerosis without residual activity | Need to attribute sclerosis to lupus nephritis rather than ischemia |
Table 2. Modified NIH Lupus Nephritis Activity and Chronicity Scoring System
| NIH Activity Index | Definition | Score |
| Endocapillary hypercellularity (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | 0-3 |
| Neutrophils/karyorrhexis (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | 0-3 |
| Fibrinoid necrosis (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | (0-3) x 2 |
| Wire loops or hyaline thrombi (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | 0-3 |
| Cellular or fibrocellular crescents (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | (0-3) x 2 |
| Interstitial inflammation (% cortex) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of cortex | 0-3 |
| Total: 0-24 | ||
| NIH Chronicity Index | Definition | Score |
| Glomerulosclerosis score, global &/or segmental (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | 0-3 |
| Fibrous crescents (% glomeruli) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of glomeruli | 0-3 |
| Tubular atrophy (% cortex) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of cortex | 0-3 |
| Interstitial fibrosis (% cortex) | None (0), < 25% (1+), 25-50% (2+), > 50% (3+) of cortex | 0-3 |
| Total: 0-12 |
Pathology of Lupus Nephritis
A wide range of pathologic changes might be observed in LN. These changes involve all compartments of renal parenchyma including glomeruli, tubules, interstation and vessels. The pathologic changes are characterized by nonprolferative, proliferative, inflammatory, and sclerotic lesions of different severities and extents. Although light microscopy examination (LM) and immunofluorescence studies (IF) provide reliable information with reasonable certainty regarding the class of LN. The EM information might provide crucial information and confirm the classification of the disease.
Class I: Minimal mesangial lupus nephritis
- Mildest form of LN with minimal clinical manifestations
- On light microscopy examination: Normal glomeruli with minimal tubulointerstitial changes
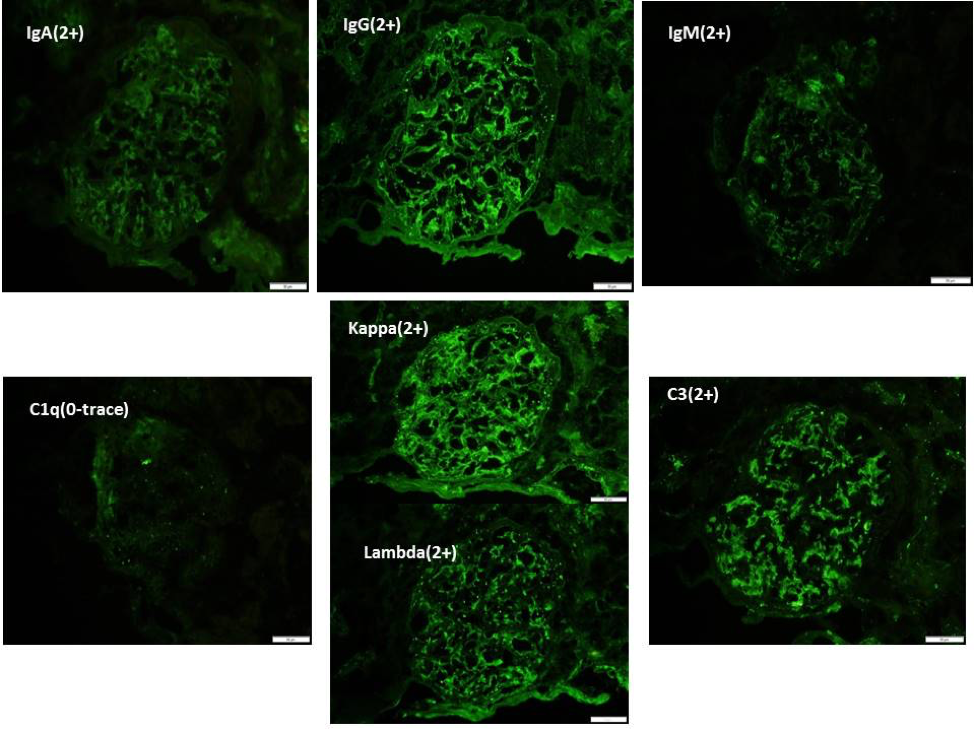
- On immunofluorescence studies: Detection of IgG deposits in the mesangial areas is essential for diagnosis. It is usually accompanied by IgA, IgM, C3, and C1q (full house). Kappa and lambda are staining equal, and IgG subclass reveals IgG1 and IgG3 predominate
- Electron microscopy helps to confirm LM and IF pictures and identify subtle findings especially in clinically “silent” SLE patients. The electron dense deposits are noted chiefly in mesangium. Tubuloreticular inclusion bodies are seen in the cytoplasm of endothelial cells
Class II: Mesangial proliferative lupus nephritis
- It accounts about 8-17% of biopsies
- Approximately 60% of the patients present with asymptotic proteinuria, or hematuria or both with preservation of renal function.
- On
by light microscopy examination:
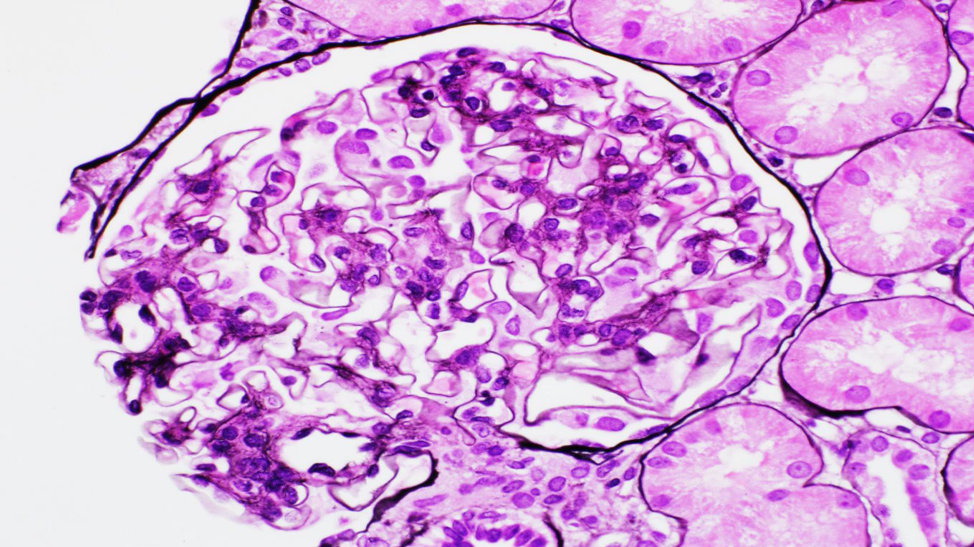
- Mesangial hypercellularity with 4 or more nuclei surrounded by matrix in mesangial area (excluding hilar area) (Figure 1)
- No endocapillary proliferation, necrosis, or crescents should present.
- On immunofluorescence studies: Full house deposits chiefly in the mesangial areas are noted ( Figure 2)
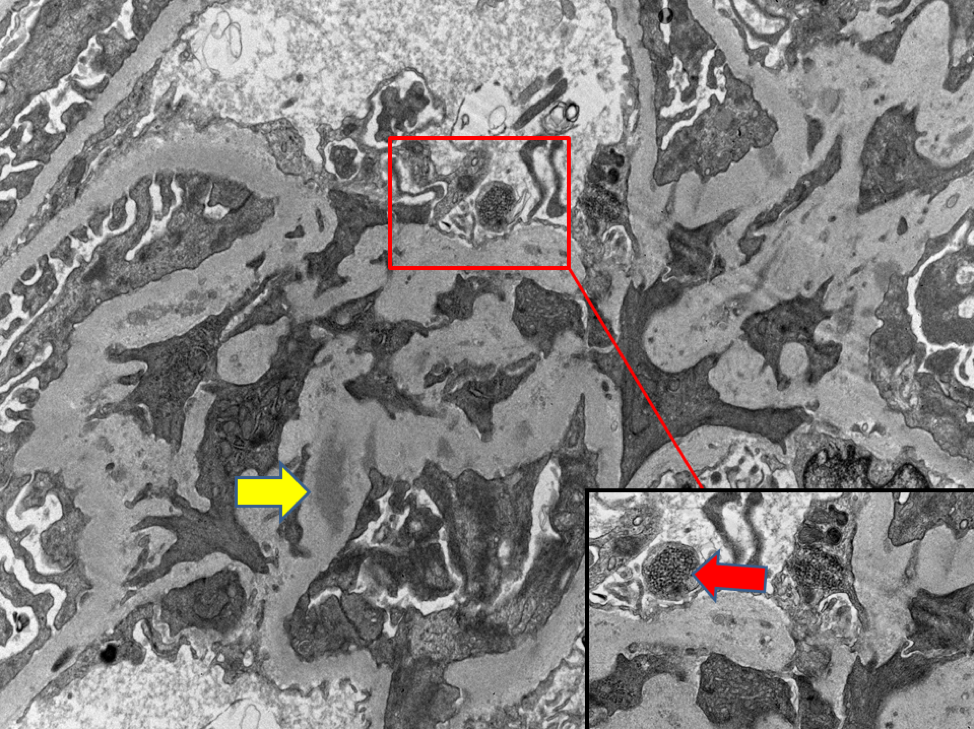
- On electron microscopy: Dense electron deposits are present predominately in the mesangium with occasional, scattered, and small subepitheial or subenotheial deposits. Tubuloreticular inclusions might be seen in the cytoplasm of endothelium (Figure 3)
Of note: The presence of confluent small endothelial deposits might increase the likelihood for transformation to higher class (like III/IV), and thus requires close clinical follow-up.
Next month, our focus on Lupus Nephritis continues with subclasses III-VI.
Sam Albadri, MBChB, M.Sc (sam_albadri)
Pathology Fellow, Mayo Clinic
References
Weening JJ et al: J Am Soc Nephrol. 15(2):241-50, 2004
Bajema et al: Kidney Int, 93:789-796, 2018
Austin et al: Kidney Int. 25:689-95, 1984.


