Understanding the basic immunologic principles is crucial for transplant management. Below is a summary of basic transplant immunology.
Major Histocompatibility Complex (MHC) Proteins
MHCs are the group of cell surface proteins which are important for self-recognition, self-tolerance and antigen-presentation. The key MHC genes are the class I genes (HLA-A, -B, and –C genes) and the class II genes (HLA-DP, -DQ, and -DR). MHC-I is expressed on all nucleated cells (except RBCs) and important for intracellular antigen presentation. MHC-II is expressed only on antigen presenting cells (APC): dendritic cells, macrophages, and B cells. MHC-II is responsible from presentation of extracellular antigens. MHC mismatch is a risk for allograft (donor organ) rejection because peptide-binding regions of the MHCs are highly immunogenic. Most immunogenic MHCs are A, B, and DR which are used as donor-recipient matching criteria for kidney transplantation.
Types of immunity
Immune system consists of mainly two subdivisions: innate (natural) and adaptive (humoral) immunity. Innate immunity is non-specific and has no memory. It is the first-line protection against foreign antigens. Adaptive immunity is antigen-specific and has memory function. Cytokines are crucial for development and differentiation for immune cells. Both type of immunities can activate each other and play role in transplant rejection. For example, ischemia-reperfusion injury during transplantation can cause expression of damage-associated molecular patterns (DAMPs) and activate innate system which cause further tissue damage and release of donor antigens. This process may result in allorecognition by B and/or T lymphocytes and subsequent allograft rejection. Similarly, allograft rejection risk increases in sepsis or infection.
Cells of Innate Immunity
- Local macrophages or dendritic cells: Both drives from monocytes. They produce the first response to foreign antigens by phagocytosis and attracts neutrophils. Dendritic cells remain local and are the most important APCs. Hence, they have a crucial role in allograft rejection. They phagocyte donor antigens and present to T-cells in lymphoid organs.
- Neutrophils: They damage microorganisms and infected cells. They don’t have antigen presenting abilities.
- Natural Killer (NK) cells: They kill the viral infected cells and tumor cells. Their killing function does not need priming by APCs. Absence of MHC-1 molecule is a signal for NK cell-induced death (which may occur in tumor cells or stressed cells) which is called “Self-missing hypothesis”.
- Other cell types include eosinophils (anti-parasitic actions), basophils / mast cell (allergic reactions, anti-helminthic) and epithelial cells.
Cells of Adaptive immunity
- T and B lymphocytes are main adaptive immune cells. They develop into self-tolerant status in the central lymphoid organs including thymus (T lymphocytes) and bone marrow (B-cells) and then migrate into peripheral lymphoid organs including lymph nodes and spleen.
- B-cells: Naïve B-cells has IgD on their surface which recognize alloantigens and undergo class switching (switch into IgG/IgE/IgA/IgM) to become a memory B-cell. This process also requires T-helper cells (Th).
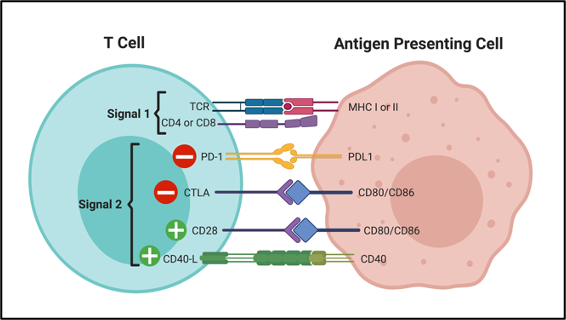
- T cells: Naïve T cells differentiate into either helper T-cell (CD4+) or cytotoxic T-cell (CD8+) in the thymus. All T-cells have T-cell receptor (TCR) and require antigen presentation by APCs to develop into memory cells. For this interaction, two signals are required: Signal 1 and signal 2 (co-stimulation). Signal 1 is the binding of TCR to MHC-antigen complex. Signal 2 is the binding of other surface molecules for activating or inhibitory co-stimulation (Figure-1).
- Cytotoxic T-cells: Naïve CD8 T-cells maturate into memory T-cells in the peripheral lymphoid organs when their TCR binds to MHC-I / antigen complex on APCs. Then, they migrate into the end-organ (allograft) and induce apoptosis of target cells by perforin/granzyme B transfer or Fas/Fas ligand binding.
- T-helper (Th) cells: There are various subtypes of T-helper cells. All of them carry CD4 molecule. Naïve CD4 cells recognize antigens in the secondary lymphoid organs when their TCR binds to MHC-II / antigen complex on APCs and differentiate into specific T-helper cells. Th1 cells activates cytotoxic T-cells and macrophages with IL-2 and IFN . Th-2 and follicular Th cells interact with B-cells and induce memory B-cell formation. Regulatory T cells (Treg) suppress the immune response and maintain self-tolerance which is important to prevent autoimmunity and transplant rejection.
How does rejection occur?
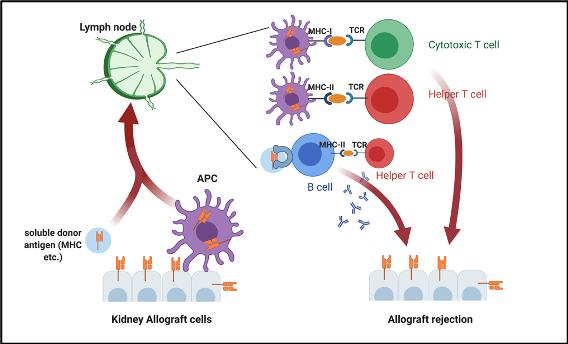
Donor’s MHC molecules are very immunogenic and are the main antigen responsible from rejection. They are captured by donor’s or recipient’s APCs and carried into the lymphoid organs. APCs present the processed antigen to cytotoxic or helper T-cells. This antigen presentation stimulates naïve T-cells to develop into memory T-cells. They migrate into graft tissue as well as secrete cytokines to recruit other inflammatory cells like macrophages to cause acute cellular rejection. Moreover, Soluble alloantigens are captured by naïve B-cells in the peripheral lymphoid organs. This induces class switching and memory B-cell formation. Memory B-cells secrete antibodies against donor’s antigens (mainly anti- MHC antibodies). Secreted antibodies opsonize the graft cells. Finally, cells with antibody-dependent cellular cytotoxicity capability (NK cells, macrophages, neutrophils, and eosinophils) and complement system attack the opsonized cells. That process causes antibody mediated rejection (Figure-2).
How are the alloantigens presented to host immune cells?
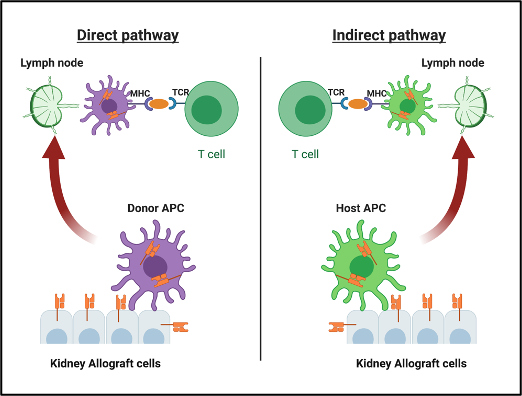
Alloantigens can be delivered and presented to T-cells through 2 different pathways (figure-3):
- Direct pathway: The transplanted organ carries number of passenger APCs in the form of interstitial dendritic cells. Direct pathway occurs when these donor’s APCs directly present their own antigens to recipient T-cells. As donor-origin APCs are depleted over time, the contribution of the direct pathway to the alloimmune response may decrease.
- Indirect pathway: This occurs when host APCs capture and present donor’s antigens to recipient T-cells.
Orhan Efe
Nephrology Fellow
Brigham Womens Hospital/Massachusetts General Hospital Program
Harvard Medical School


