Welcome to the 12th case of the Skeleton Key Group, a team of forty+ nephrology fellows who work together to build a monthly education package for Renal Fellow Network. The cases are actual cases (without patient identifying information) that intrigued the treating fellow.
Written by: Varun Mamidi
Visual Abstract by: Namrata Parikh
A. The Stem
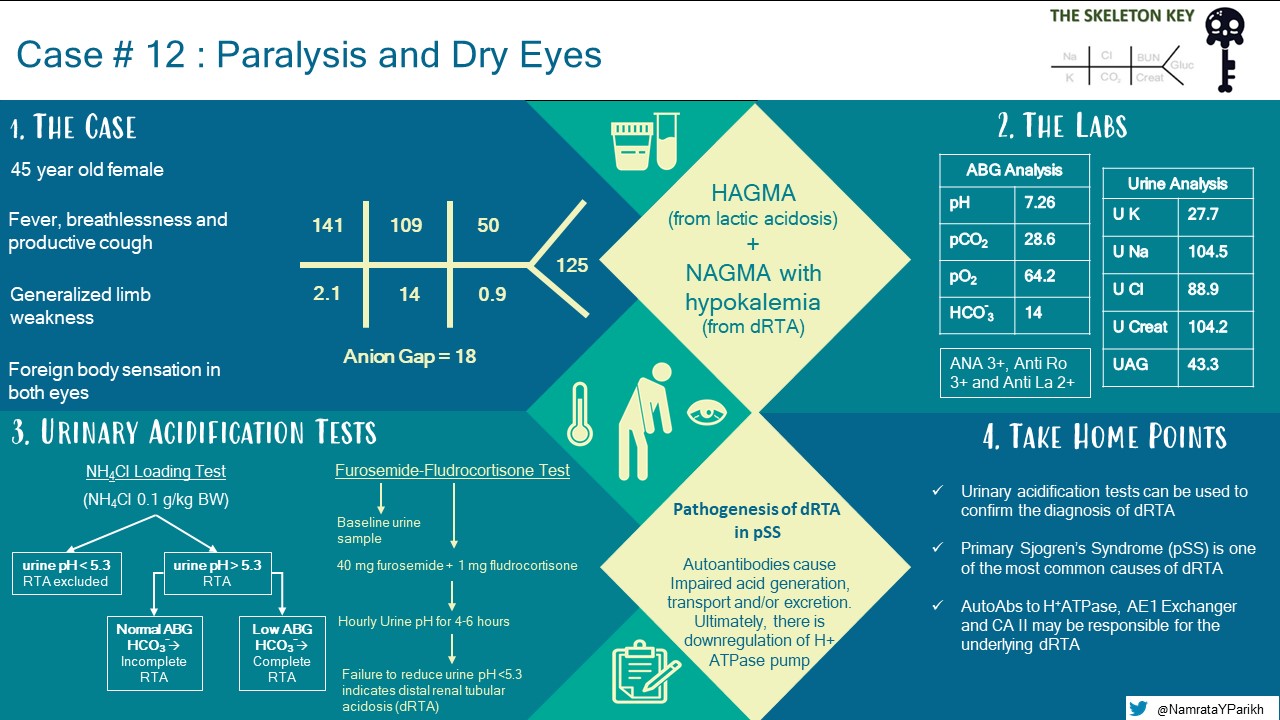
A 45-year-old female with a past history of hypothyroidism presented to the emergency department with five days of fever, productive cough, breathlessness and weakness involving all four limbs. She also presented with foreign body sensation and dryness in her both eyes for the last 6 months. Currently, her home medication was thyroxine 25mcg once daily.
Vitals: Blood pressure: 120/70 mmHg, Heart rate: 98/min, Temperature: 98.6 oF (Oral), Respiratory rate: 26/min and O2 Saturation: 96% with O2 at 2 L/min. Weight: 62 kg, Height: 160 cm, BMI: 24.2 kg/m2.
Physical examination:
General: Alert and oriented
Head/Ears/Eyes/Nose/Throat: Normal. No scleral icterus
Pulmonary: Bilateral basal crepitations present
Cardiovascular: Normal S1 and S2. No murmurs
Abdomen: Soft, non-tender
Extremities: Normal
Nervous system: Motor power of 3/5 in bilateral lower limbs and upper limbs. Bilateral deep tendon reflexes were absent and bilateral plantars were flexor. Higher mental functions, cranial nerves, sensory examination and cerebellar functions were normal
B. The Labs
Provisional diagnosis for weakness:
1) Pneumonia
3) Metabolic acidosis
4) Hypothyroidism
Nephrology was consulted for diagnostic evaluation and management of hypokalemia.
C. Differential Diagnosis: Hypokalemia in Metabolic Acidosis
When evaluating hypokalemia, the first step is to look for any symptoms suggestive of an emergency that requires immediate therapy. This includes cardiac arrhythmias/ECG changes and muscle weakness. In our patient, the presence of severe hypokalemia and sudden onset of muscle weakness supports the diagnosis of acute hypokalemic paralysis and hence, she was given emergency treatment with intravenous potassium chloride.
We then follow a systematic approach (Algorithm 1).
First we rule out pseudohypokalemia. Pseudohypokalemia is caused by time-dependent K+ uptake by cells due to prolonged sample storage at increased ambient temperature after venipuncture. It is commonly seen in patients with acute leukemia. Rapid segregation of plasma and storage at 4oC is done to avoid this artifact.
Once pseudohypokalemia is ruled out, causes of hypokalemia can be grouped according to three discrete buckets.
1. Decreased filling of bucket (low intake)
2. Bucket that is leaky all over (transcellular shift)
3. Bucket with the drain wide open all the time (urinary or GI loss)
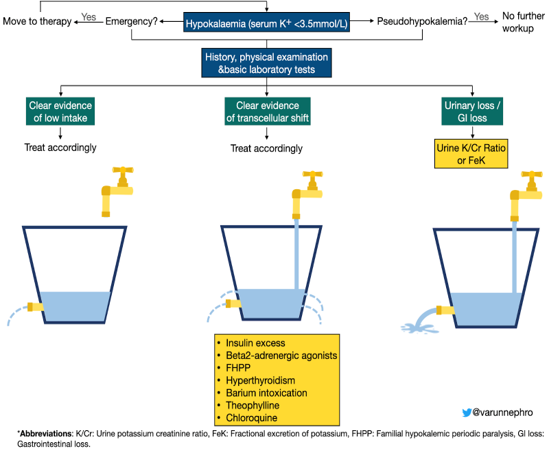
Algorithm 1: Diagnostic algorithm for hypokalemia. Adapted from Brenner & Rector’s The Kidney 11th Edition.
Our patient had no evidence of low oral intake, transcellular shift, or GI losses, and so we investigated urinary loss. The below urine and serum investigations were used to assess urine potassium to creatinine ratio.
Based on the above urine and serum investigations:
Urine potassium-to-creatinine ratio (K/Cr) (mEq/g)
= 27.7 mEq/L / 104.2 mg/dL = 27.7 mEq/L / 1042 mg/L
= 27.7 mEq/L / 1.042 g/L = 26.5 mEq/g
(K/Cr > 15 mEq/g suggests urinary loss of potassium)
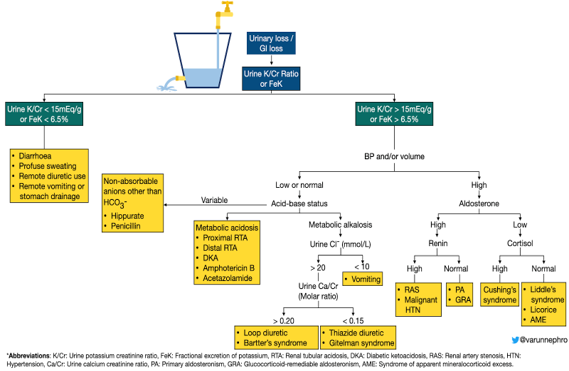
Algorithm 1, continued: Diagnostic algorithm for hypokalemia. Adapted from Brenner & Rector’s The Kidney 11th Edition.
The urine K/Cr result indicates that the cause of hypokalemia in our patient is due to kidney loss of potassium in the setting of a high anion gap metabolic acidosis (AGMA) combined with a normal anion gap metabolic acidosis (NAGMA) with appropriate respiratory compensation. The high anion gap can be explained by lactic acidosis due to sepsis, but, what is the cause for NAGMA? You can use the mnemonics HARDUP or USEDPART to assess for the causes of NAGMA as mentioned in our previous post, but given the urinary potassium wasting, we were concerned about a renal tubular acidosis.
Why did we choose to assess urine K/Cr ratio instead of TTKG or FeK? Review Case 2 to find out why!
For more information on how to process the acid-base status, see solving acid/base problem (pLACO) by NephSim
What do HARDUP or USEDPART stand for? Review Case 7!
If you recall, we utilize either a urine anion gap and/or urine osmolar gap to determine if the kidney is handling acid appropriately in the setting of acidosis. If you recall from our first case, the urine osmolar gap is a better estimation of urinary ammonium (ie acid secretion). We calculated our urine anion gap (UAG) to be 43.3 mEq/L.
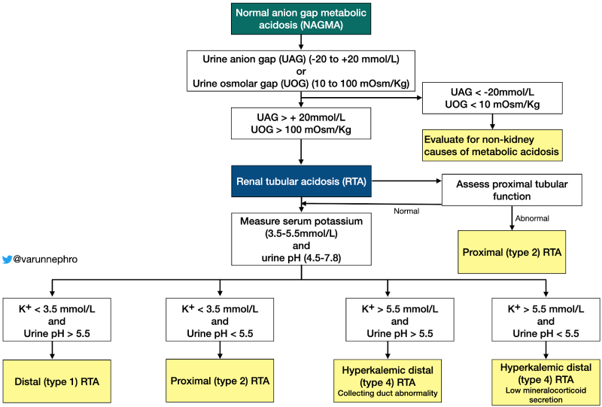
Non-anion gap metabolic acidosis, a positive UAG, and hypokalemia support a diagnosis of either a proximal or distal RTA. The mechanism of hypokalemia in distal RTA is not fully elucidated, but likely results from increased urinary potassium loss from tubular damage, decreased proximal tubular potassium reabsorption due to acidemia and increased urinary sodium loss resulting in relative hypovolemia and aldosterone stimulation, which increases urinary potassium loss.
Our patient has no features suggestive of proximal tubular dysfunction such as euglycemic glycosuria, phosphaturia, uricosuria and aminoaciduria, and so this supports the diagnosis of distal (type 1) RTA as the cause of NAGMA in our patient.
D. More Data (Confirmatory Testing)
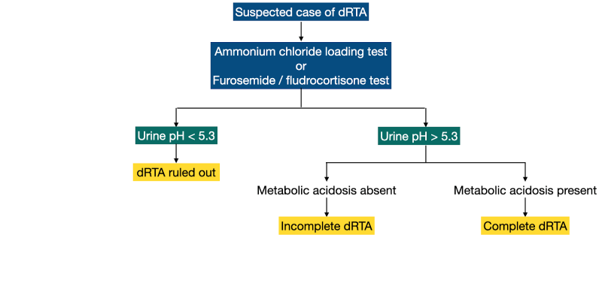
Distal RTA (dRTA) is characterized by impairment of urinary acidification and is associated with hypokalemia, nephrolithiasis, and nephrocalcinosis. In some cases, there is no metabolic acidosis because the impairment of urinary acidification is not severe, termed incomplete dRTA. Incomplete dRTA may be suspected in the setting of sustained urine pH >5.5 plus unexplained hypokalemia, nephrocalcinosis, or recurrent calcium stones.
Confirmatory testing for complete or incomplete dRTA involves provoking maximal urinary acidification then measuring urine pH (Algorithm 3). These tests include:
1) Ammonium chloride-loading test
2) Furosemide/fludrocortisone test
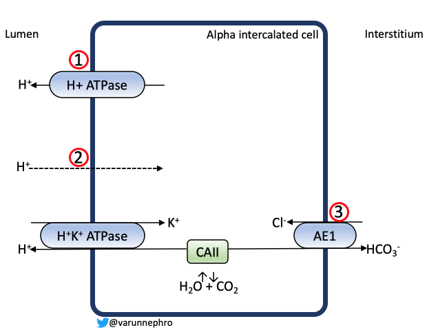
Understanding the pathophysiology of distal RTA can help solidify why the confirmatory tests are helpful. Imparied acid secretion occurs through one of the following mechanisms (Figure 1)
- Secretory defect in impaired H+-ATPase function/activity in type A intercalated cells
- Gradient defect resulting from increased permeability of the apical membrane of the type A intercalated cell to H+. This allows H+ in the tubular fluid to leak back into the cell and is seen in certain medications such as amphotericin B.
- Defects in basolateral anion exchanger (AE1) results in intracellular accumulation of HCO3–, which limits H+ secretion.
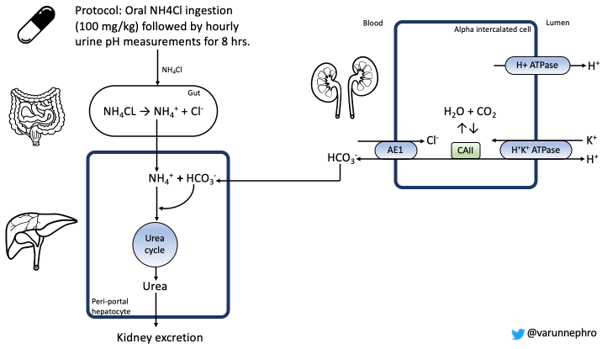
The ammonium chloride loading test (Figure 2) is considered the “gold standard” for the diagnosis of dRTA. It was first described by Wrong and Davis in 1959 as a short ammonium chloride test. Ingestion of ammonium chloride gives the patient an “acid load,” and the kidney responds through excretion of H+ in the distal tubule. Failure of the urine pH to acidify is confirmatory.
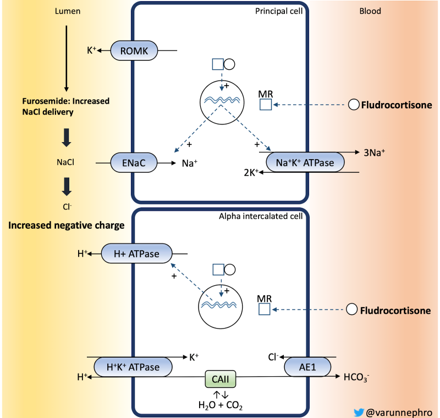
Furosemide / fludrocortisone test:
The furosemide / fludrocortisone test was initially described in 1986 as the oral furosemide test by Dan Batlle. SB Walsh found that the simultaneous administration of furosemide and fludrocortisone is as potent a stimulus for urinary acidification as the metabolic acidosis caused by the NH4Cl loading test by increasing delivery of Na+ to the cortical collecting duct, resulting in uptake by the principal cell and a “negative” charge in the tubular fluid. This negative charge is the stimulus for acid efflux.
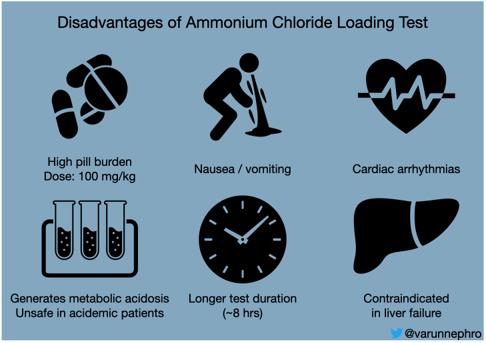
Though NH4Cl loading test is considered as the gold standard in the diagnosis of distal RTA, it has its disadvantages (Figure 4). Compared to the NH4Cl loading test , the Furosemide/Fludrocortisone test is safe, has fewer side effects, reduced pill burden, and has shorter test duration (~ 6 hours). Learn more about the renal acidification tests from ‘Distal Renal Tubular Acidosis Developments in its Diagnosis and Pathophysiology’ by Stephen Benedict Walsh.
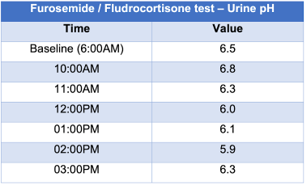
In this patient, Furosemide/fludrocortisone test (Figures 5 and 6) was done to confirm the diagnosis of distal RTA. As the urine pH was consistently above 5.3 throughout the duration of the test, the diagnosis of distal (type 1) RTA was confirmed.
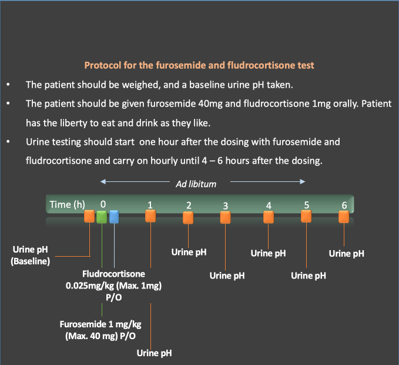
The results of furosemide/fludrocortisone test were as follows:
E. Final Diagnosis
E. Final Diagnosis
There are many causes of distal RTA, but in our patient, further serologic testing for ANA, anti-Ro/SSA, anti-La/SSB were positive along with the Schirmer’s test. A diagnosis of primary Sjogren’s syndrome was confirmed. The incidence of kidney manifestations in primary SS is <10% and usually has a favourable prognosis. Tubulointerstitial nephritis (TIN) is the most frequent renal manifestation. Distal RTA due to primary Sjogren’s syndrome is suspected to be from autoantibodies to H+ATPase, AE1 exchanger as well as carbonic anhydrase II. See this tweetorial for more.
F. Management
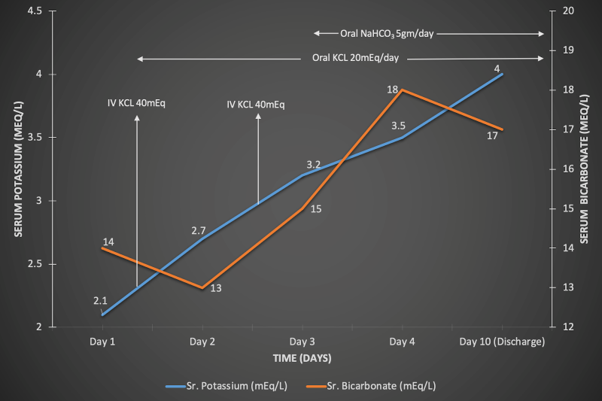
So let’s summarize. Our patient’s weakness was attributed to her hypokalemia and metabolic acidosis, which were related to a distal RTA from primary Sjogren’s disease. She was treated with IV and PO potassium with improvement in her quadriparesis and oral bicarbonate therapy. It is imperative to start alkali therapy after potassium correction to avoid worsening the hypokalemia from transcellular shift.
Her pneumonia recovered with antibiotic therapy, and her extrarenal manifestations of Sjogren’s were managed with symptomatic therapy and did not require immunosuppressants or DMARDs. At 3 month follow up, the patient had no further episodes of hypokalemia and paralysis.
G. Take-Home Points
- It is important to replete potassium as soon as possible before workup in patients with ECG changes, severe hypokalemia (<2.5mEq/L) and the presence of muscle paralysis.
- Tests for renal acidification such as ammonium chloride loading test or furosemide/fludrocortisone test can be used to confirm the diagnosis of distal RTA.
- pSS is one of the most common causes of distal (type 1) RTA, but chronic TIN is the most common renal manifestation of pSS.
- Autoantibodies to vacuolar H+-ATPase, AE1 exchanger and CA II are the proposed mechanisms for distal RTA in pSS.



I’m wondering could we use urine AG to evaluate kidney is handling acid appropriately under the setting of HAGMA?
excellent explanation with well balanced combination of problem oriented approach and utilization of knowledge of renal physiology.
thanks to the team.
Excellent presentation, wil use it for my students