Welcome to the first case of the Skeleton Key Group, a team of twenty odd nephrology fellows who work together to build a monthly education package for Renal Fellow Network. The cases are actual cases that intrigued the treating fellow.
Case 1: Written by Prakash Gudsoorkar
Check out Sayna Norouzi’s video on this case here.
There’s more! A #tweetorial from Amy Yau here.
A. The Stem
A 30 year old woman with sickle cell anemia, previously treated with hydroxyurea, and HIV on antiretroviral therapy. Her last CD4+ cell count was 410 cells/microL but that was two years ago. She is now admitted with acute onset abdominal,chest and bilateral shin pain. Her admission diagnosis is sickle cell pain crisis.
Her current medications: tenofovir alafenamide, lamivudine, efavirenz, hydromorphone as needed. She denies taking any over-the-counter medications. Her pain crisis is being managed with high flow oxygen and hydromorphone.
B. The Labs
On the day of admission…
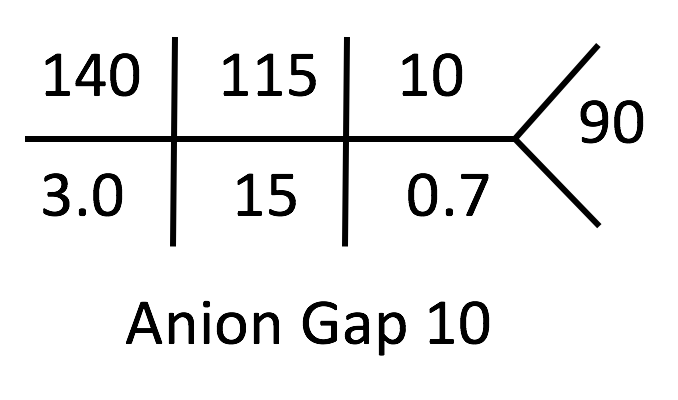
| Test | Value |
| Sodium | 140 mmol/L |
| Potassium | 3.0 mmol/L |
| Bicarbonate | 15 mmol/L |
| Chloride | 115 mmol/L |
| Blood urea nitrogen | 10 mg/dL |
| Creatinine | 0.7 mg/dL |
| Blood glucose | 90 mg/dL |
| CKD-EPI eGFR | 78 mL/min/1.732 sq m. |
Nephrology is consulted for suspected metabolic acidosis and hypokalemia.
How will you proceed?
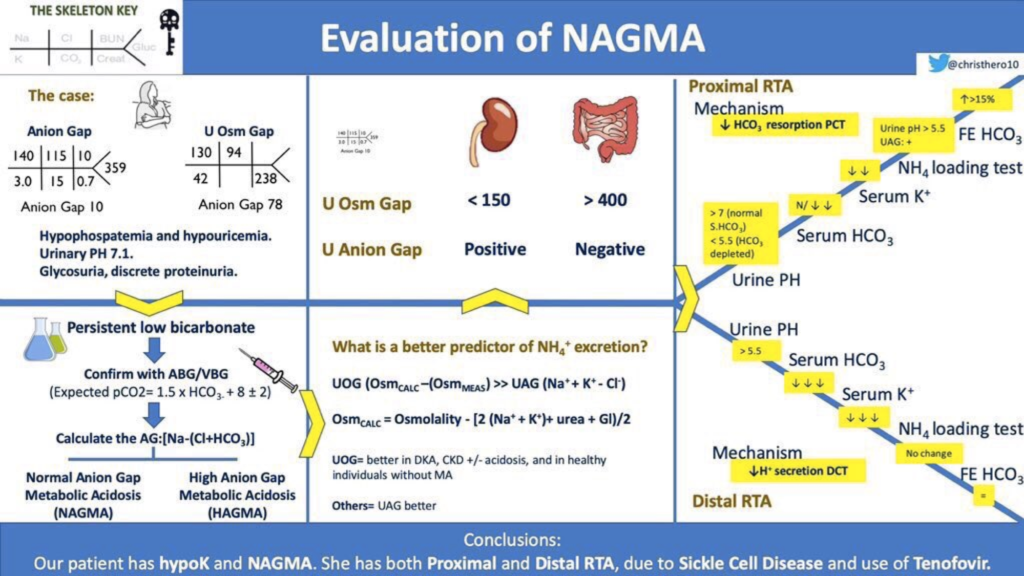
The first step should be to perform an arterial or venous blood gas (ABG/VBG), to confirm metabolic acidosis.
| pH | 7.38 |
| pCO2 | 30 mm of Hg |
| pO2 | 138 mm ofHg (on 5L O2 by nasal prongs) |
| HCO3 | 14 mmol/L |
Metabolic acidosis confirmed.
According to Winter’s formula (expected pCO2 = 1.5 x HCO3 + 8 ± 2 = 29 ± 2), we have appropriate respiratory compensation
C. First Diagnosis: Primary metabolic acidosis with appropriate respiratory compensation
The next step is calculating the anion gap:
Na – (Cl + HCO3) = 140 – (115 + 15) = 10
As the patient’s albumin is normal, we have normal anion gap metabolic acidosis (NAGMA) with hypokalemia. The most common cause of this will be diarrhea. Patient has abdominal symptoms so this is a reasonable diagnosis…but the patient did not have diarrhea.
Next on the differential for NAGMA and hypokalemia would be a renal tubular acidosis (RTA). The patient has a couple risk factors for RTA. Tenofovir can cause a proximal RTA (almost always associated with hypophosphatemia) and sickle cell disease can cause a distal RTA.
Here’s a mnemonic for etiologies of NAGMA:
USED PART
U: Uretero enterostomy
S: Small bowel fistula
E: Extra chloride
D: Diarrhea
P: Pancreatic fistula
A: Addison’s disease, acetazolamide
R: RTA
T: Tenofovir and topiramate
Let’s drill down and look at urine anion gap (UAG).
In response to metabolic acidosis, healthy kidneys ramp up excretion of hydrogen ions. Since urine pH can’t go much lower than a pH of 5 (0.001 mmol/L), to meaningfully increase hydrogen ion excretion the hydrogen is bound to titratable acid (primarily dihydrogenphosphate, H2PO4-) and ammonium (NH4+).
Urinary phosphate is largely limited by dietary phosphate, while ammonia can be generated from the metabolism of glycine and can be upregulated in response to acidosis. Because of this, tracking urinary ammonium is a good way to check to see if the kidneys are responding appropriately to metabolic acidosis. The more the ammonium in the urine, the lower the UAG will go. In a distal RTA, the UAG is positive due to impaired excretion of ammonium in the urine and is a good surrogate for impaired ammonium excretion. The UAG, like the serum anion gap, identifies an abnormal ion suspected to be either present (like lactate or ketones in a serum anion gap) or inappropriately low (like ammonium in a urinary anion gap)
How is the UAG = (Na+ + K+) – Cl– formula derived?
At a given time, to maintain electroneutrality…
Urine Anions = Urine Cations
(Cl– + HCO3–) + unmeasured anions = (Na+ + K+) + unmeasured cations
Unmeasured anions – unmeasured cations = (Na+ + K+) – (Cl– + HCO3–)
Urine [HCO3– ] can be considered to be negligible at urine pH < 6.6. Thus, bicarbonate can be deleted from the equation. The UAG is equal to the difference of the unmeasured anions and unmeasured cations, so:
UAG = (Na+ + K+) – Cl–
Interpretation of the UAG:
- Positive UAG (generally between 20 and 90 mEq/L) is usually indicative of low ammonium excretion. Patients with metabolic acidosis due to impaired renal NH4 excretion (such as a distal RTA) will have a positive UAG.
- Negative UAG (generally between -20 and -50 mEq/L) is usually indicative of increased ammonium excretion (ie, greater than 80 mEq/L). Such values of the UAG occur in patients with metabolic acidosis generated by diarrhea.
- Results near zero (ie, between +20 and -20) cannot be reliably interpreted.
Limitations of the UAG:
- Presence of keto-anions increase the UAG.
- Presence of high lithium decrease the UAG.
- The ability to excrete ammonium is markedly curtailed with decreased kidney function. In addition, confounding variables related to concurrent alterations in unmeasured anions (sulfate and phosphate) further interfere with the UAG – ammonium relationship in CKD. Reduced nephron number, increasing plasma potassium, and reduced aldosterone (due to RAAS blockers, limit the ability to excrete ammonium in CKD, even in the presence of acidosis.
Unexpected anions (like ketones, bicarbonate, or hippuric acid) can mask ammonium and “fool” the urinary anion gap in giving the false impression of an RTA where none exists.
The diagnostic fallacies of UAG led to introduction of the urine osmolal gap (UOG) as a surrogate marker for urine ammonium excretion. So let’s take a look at this UOG.
Due to the previously mentioned limitations of the UAG, the UOG has gained acceptance. Just as the serum osmolar gap allows one to detect the presence of an unmeasured osmole (a toxic alcohol) by comparing the measured and calculated osmolarities, the urinary osmolar gap allows one to detect unmeasured urinary ammonium.
The UOG is calculated as:
U osm – [2 x (U Na + U K) + U glucose (mg/dL) /18 + U urea nitrogen (mg/dL)/2.8]
A normal urine UOG is approximately 10 to 100 mOsm/kg, with urinary ammonium excretion being approximately one-half of this value (5-50 mmol/L) due to accompanying anions. In conditions of metabolic acidosis, if ammonium concentration is high, (e.g. urine osmolal gap > 200), a non-kidney cause for the acidosis (like GI losses) is more likely than a RTA.
Advantages of the UOG:
An advantage of estimating urine osmolal gap over urine anion gap is that the UAG may be misinterpreted when ammonium may be expected to be excreted with a non-chloride anion, such as beta-hydroxybutyrate or acetoacetate (diabetic ketoacidosis) or hippurate (toluene intoxication).
Limitations of the UOG:
1. The presence of urease producing bacteria will disrupt the relationship between UOG and kidney NH4 excretion.
2. Urinary excretion of osmotically active, non-ammonium solutes (alcohols and mannitol) will increase the UOG even when kidney ammonium excretion is not high.
3. The UOG will decrease if urine Na and K do not completely dissociate from their respective anion. Dissociation of sodium and potassium salts is inversely related to the urine osmolality; therefore, the UOG may underestimate kidney NH4 excretion when the urine is highly concentrated.
(Courtesy: Joel Topf, presented at KIDNEYcon)
Here, the UOG could not be calculated as all urine chemistries were not available in this case.
D. The Urinalysis & Urine Chemistry
| Test | Value |
| Na | 130 mmol/ L |
| K | 42 mmol/ L |
| Cl | 94 mmol/ L |
| pH | 7.1 |
| Glucose | 2+ |
| Protein | 100 mg/dL |
| Protein to creatinine ratio | 0.42 mg/mg |
In this case, the NAGMA with an elevated urinary potassium support RTA.
The urinary anion gap (Na+K-Cl = 130+42-94 = 78) reveals a high number of unmeasured anions in the urine, making the urinary excretion of additional unmeasured cations (think ammonium) unlikely.
After calculating the urinary anion gap, this looks like a Renal Tubular Acidosis (RTA).
How do we tell proximal & distal RTAs apart?
In a distal RTA, expect the distal urinary pH is always > 5.3 & the urinary AG to be positive.
In proximal RTA, look for a urinary pH < 5.3 and negative urinary AG as the ability to excrete H+ is intact. However, treatment with bicarbonate can lead to an elevated urine pH and positive urinary anion gap (from anionic bicarbonate).
Here, the urinary AG positive + urinary PH 7.1 –> THINK distal RTA.
This patient likely has distal RTA which could possibly be due to sickle cell disease. The patient also has glycosuria and proteinuria…which suggests proximal RTA.
E. More Data
| Test | Value |
| Serum Uric acid | 2.1 mg/dL |
| Serum Phosphate | 2.9 mg/dL |
Hypophosphatemia and hypouricemia support proximal RTA…but we have a negative UAG…which is less common in proximal RTA unless the patient is receiving bicarbonate…
Imaging of the abdomen reveals bilateral nephrocalcinosis. A urine calcium/creatinine ratio is 0.21 mg/mg. Patients with distal renal tubular acidosis do have hypercalciuria. This may be due to constant release of calcium phosphate from bones to buffer the extracellular H+ as well as decreased reabsorption of calcium and phosphate, leading to hypercalciuria and hyperphosphaturia.
Nephrocalcinosis in distal RTA is predominantly due to following main reasons:
- Patients have relatively alkaline urine, which promotes calcium phosphate precipitation.
2. Metabolic acidosis and hypokalemia lead to hypocitraturia, a risk factor for stones. Citrate in the urine complexes calcium and inhibits stone formation.
F. The Answer
After analyzing the entire case, it looks like this patient has TWO different RTAs:
1. Proximal RTA, due to tenofovir +/- sickle cell disease.
Glycosuria: due to kidney glucose wasting
Hypophosphatemia: due to kidney phosphate wasting
Hypouricemia: due to kidney uric acid wasting.
2. Distal RTA, due to sickle cell disease
Positive urinary anion gap: indicative of impaired renal ammoniagenesis.
Nephrocalcinosis.
High urine calcium : creatinine ratio.
References:
1. Daniel Batlle, Sheeba Habeeb Ba Aqeel et al. The Urine Anion Gap in Context CJASN 2018. 13: 195–197.
2. Daniel Batlle, JamieChin-Theodorou et al Metabolic Acidosis or Respiratory Alkalosis ? Evaluation of a Low Plasma Bicarbonate Using the Urine Anion Gap. AJKD Volume 70, Issue 3, September 2017, Pages 440-444. 3. Mandana Rastegar, MD, EdM, and Glenn T. Nagami, MD Non–Anion Gap Metabolic Acidosis: A Clinical Approach to Evaluation. Am J Kidney Dis. 2017; 69 (2):296-301.
4. Karl A. Nath and Robert P. Hebbel Sickle cell disease: renal manifestations and mechanisms. Nat. Rev. Nephrol. 2015 Mar; 11(3):161-171.
5. Raphael KL, Gilligan S, Ix JH: Urine anion gap to predict urine Ammonium and related outcomes in kidney disease. ClinJAmSoc Nephrol 13: 205–212, 2018.



This is outstanding.
great effort thank you
Congratulations!!! excellent work !!!