Welcome to the 16th case of the Skeleton Key Group, a team of 40-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network. The cases are actual cases (without patient identifying information) that intrigued the treating fellow.
Written by: Jefferson L Triozzi, MD
Visual abstract by: Sai Achi, MD
A. The Stem
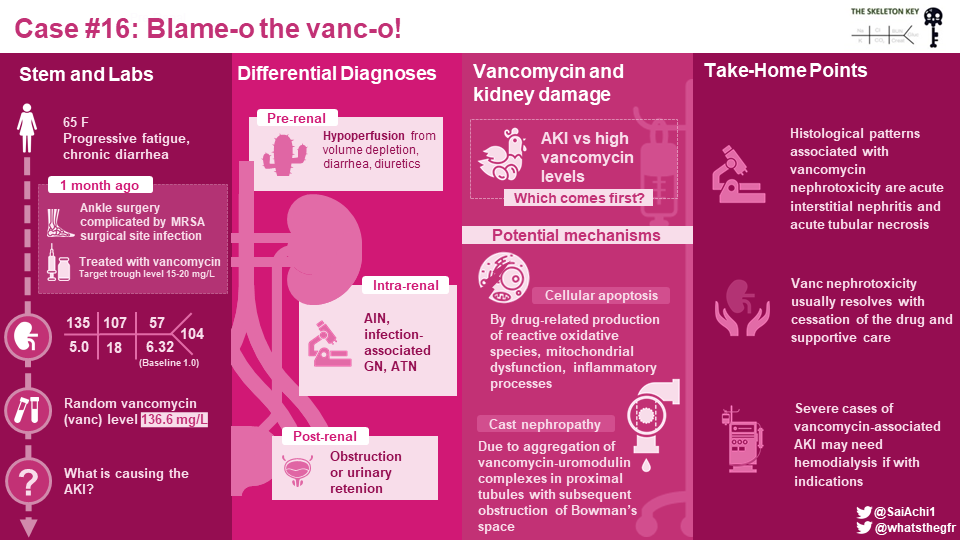
A 65-year-old woman with type two diabetes mellitus, hypertension, dyslipidemia, obesity and right ankle osteomyelitis on six weeks of intravenous vancomycin presented with progressive fatigue and malaise.
One month prior to presentation, the patient underwent a right ankle ligament reconstruction. The surgical site became infected, requiring surgical debridement and hardware removal. Intraoperative cultures grew methicillin-resistant Staphylococcus aureus (MRSA) and she was started on intravenous vancomycin for osteomyelitis. She underwent therapeutic drug monitoring in the outpatient setting.
Two weeks prior to presentation, she received routine bloodwork with a reportedly normal complete blood count, comprehensive metabolic panel, and vancomycin trough level of 16 mg/L (within a goal of 15-20 mg/L).
On presentation, she described progressively worsening fatigue over the preceding week. She denied fever, chills, nausea, vomiting, chest pain, shortness of breath, or poor intake of food and drink. No dysuria, urinary hesitancy, or urinary urgency. She reported one to two loose stools daily since starting the antibiotic. Her vital signs were stable (temperature 98.6 oF, blood pressure 162/74 mm Hg, heart rate 79 beats per minute, respiratory rate 14 breaths per minute). Her physical examination was notable for morbid obesity (BMI 40) and a partially healed surgical site on the right ankle. Her extremities were warm and dry, with no evidence of infection or volume overload.
Medications:
vancomycin 1.25 g IV Q12 hours (day 28)
lisinopril-hydrochlorothiazide 20-25 mg daily
metformin 500 mg twice a day
atorvastatin 40 mg nightly
multivitamin tablet daily
naproxen 500 mg as needed not more than twice a day
B. The Labs
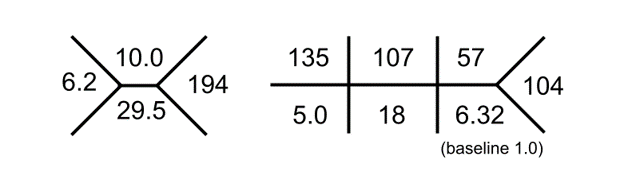
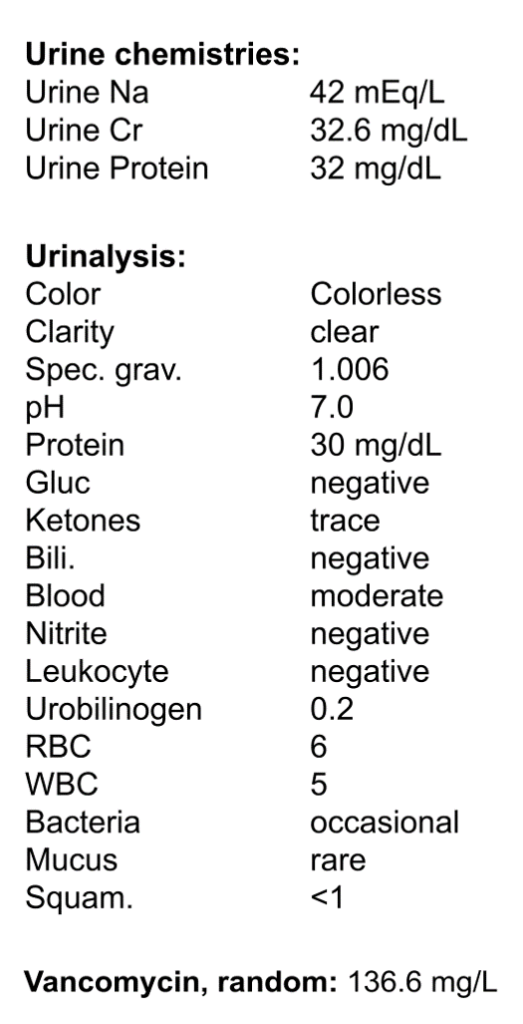
C. Differential Diagnosis
The initial workup revealed acute kidney injury and an exceptionally elevated random vancomycin level of 136.6 mg/L (drawn 10 hours after the last vancomycin infusion). Vancomycin nephrotoxicity was immediately suspected.
Vancomycin nephrotoxicity—a challenging diagnosis
Approved in 1958 for the treatment of gram-positive bacterial infections, Vancomycin nephrotoxicity is a challenging diagnosis because elevated vancomycin levels are often an effect of acute kidney injury, not the cause.
Diminished kidney function causes elevated vancomycin levels
Vancomycin is primarily eliminated by glomerular filtration (90%) and, to a lesser extent, secretion in the proximal tubule (10%). Vancomycin clearance is directly proportional to glomerular filtration rate (Figure 1). A comprehensive evaluation is therefore important to rule out alternative causes of acute kidney injury in the setting of elevated vancomycin levels. Patients receiving vancomycin often have many alternative reasons for decreased kidney function, including acute illness, hypovolemia, infection, or concomitant nephrotoxic agents.
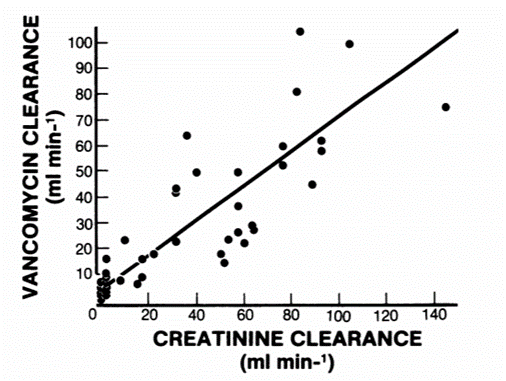
The general approach to acute kidney kidney injury includes hemodynamic, intrinsic renal, and post-renal etiologies. In this patient, causes of acute kidney injury to consider other than vancomycin nephrotoxicity include pre-renal (hypovolemia, sepsis, antibiotic-associated diarrhea, thiazide diuretic), intrinsic renal (acute tubular injury, infection-associated glomerulonephritis, interstitial nephritis related to medications), and post-renal (urinary retention or obstruction).
Vancomycin causes acute kidney injury
There is a “modest” association between vancomycin and acute kidney injury. Vancomycin nephrotoxicity is a diagnosis of exclusion. There is no universally accepted diagnostic criteria or specific serum vancomycin concentration level used in the diagnosis (as noted above). Risk factors include obesity, underlying chronic kidney disease, hypovolemia, acute illness/critical illness, and administration of concomitant nephrotoxic agents. For example, vancomycin in combination with piperacillin/tazobactam is associated with three times the odds of nephrotoxicity compared to vancomycin alone. The incidence of acute kidney injury also increases with greater daily dosage (>4g vancomycin/day), treatment duration (≥1 week), and higher goal vancomycin trough levels (>15 mg/L). Often patients with serious infections require higher vancomycin concentrations to be effective (e.g., infections of bone, central nervous system, lung, bloodstream). Prolonged exposure to high vancomycin levels increases the risk of permanent kidney failure.
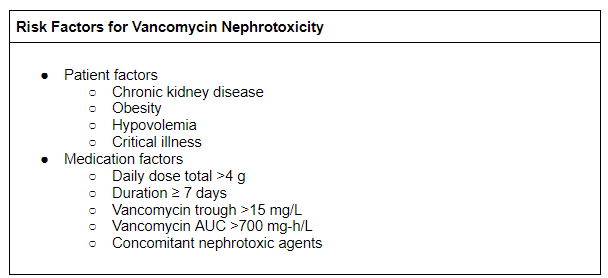
Our patient has numerous risk factors for vancomycin nephrotoxicity. Due to the deep-seated nature of infection, the patient is receiving vancomycin for a long duration (six weeks) with a high goal vancomycin trough (15-20 mg/L). She is also prone to hypovolemia, as she is taking a thiazide diuretic and also has antibiotic-associated diarrhea. The patient is obese, which portends greater risk of drug toxicity due to a larger volume of distribution and a greater dose requirement based on actual body weight. Finally, she takes NSAIDs for low back pain; concomitant nephrotoxic agents are associated with an increased risk of vancomycin nephrotoxicity.
Dosing and Monitoring
Vancomycin dosing is based on baseline kidney function and actual body weight. Appropriate dosing and monitoring are important to establish safe and effective vancomycin levels, particularly in high risk patients (Table 2).
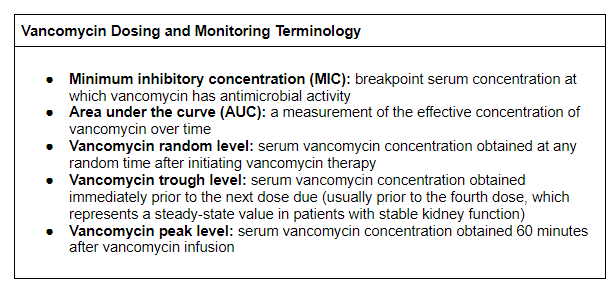
Table 2. Vancomycin dosing and monitoring terminology.
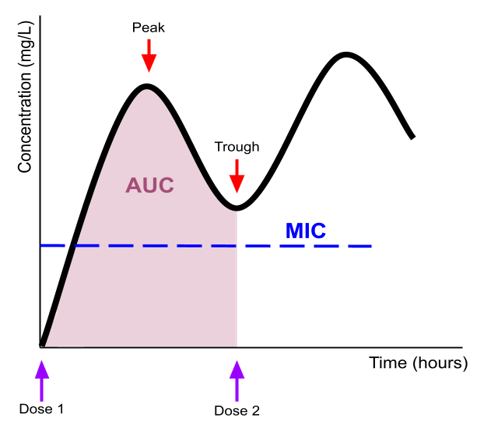
Vancomycin antimicrobial activity is both time- and concentration-dependent, which is conceptualized as the Area Under the Curve (AUC) (Figure 2). Vancomycin serum levels must exceed a Minimum Inhibitory Concentration (MIC) required for effective antimicrobial activity, which is measured with the bacterial culture. Unfortunately, increasing microbiological resistance to vancomycin has led to greater dosage requirements and likely greater nephrotoxicity (a phenomenon known as “MIC creep”). In fact, if MIC is greater than 1 mg/L, an alternative antibiotic should be used because the amount of vancomycin required will pose too much nephrotoxicity risk.
There are two strategies to dose vancomycin.
- Firstly, AUC-guided dosing involves checking 1-2 vancomycin levels to predict the AUC using pharmacokinetic modeling or Bayesian estimation computer software, then adjusting dosing based on the predicted AUC. AUC is the preferred method of vancomycin dosing per the new 2020 clinical practice guidelines, but has not been widely adopted because many healthcare systems do not own the computer software.
- A second method, trough-only dosing, uses serial measurements of vancomycin trough levels to guide the dosing regimen. Trough-only monitoring was recommended by the old 2009 clinical practice guidelines and, although technically no longer recommended, is still widely used in clinical practice! A goal vancomycin trough of 15 to 20 mg/L is usually recommended for serious MRSA infections (e.g., infections of bone, central nervous system, lung, bloodstream). Vancomycin trough levels should be obtained in the steady-state, typically prior to the fourth dose and once weekly thereafter in patients with stable kidney function.
Notably, AUC-guided dosing has been associated with less risk of acute kidney injury than vancomycin trough-only dosing.
Therapeutic drug monitoring is standard practice for patients receiving vancomycin therapy to ensure therapeutic drug concentrations and monitor for drug toxicities. Vancomycin may cause ototoxicity (associated with high vancomycin level) and rarely cytopenias (independent of vancomycin level). The characteristic “red man” syndrome is an infusion reaction that more often occurs with rapid administration of the first dose of vancomycin, and is less likely with subsequent doses. In this case, the patient received therapeutic drug monitoring after discharge with reportedly normal laboratory results. Although therapeutic drug monitoring decreases the risk of nephrotoxicity, a small percentage of patients develop toxicities despite close monitoring.
D. More Data
In this case, kidney biopsy was pursued to clarify the diagnosis because the patient’s kidney function continued to worsen despite cessation of vancomycin, fluid resuscitation, and supportive care. Kidney biopsy is not routinely warranted in the evaluation of vancomycin nephrotoxicity. Further serologic workup (including blood cultures, HIV and hepatitis virus panels, creatine kinase) and kidney ultrasound were unremarkable. The biopsy demonstrated acute tubular epithelial injury, morphologically consistent with acute tubular necrosis (Figures 3 and 4).
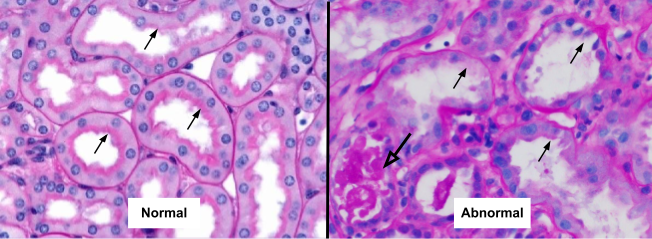
Figure 3. Periodic Acid of Schiff (PAS) stain x400 amplification. The PAS reagents stain the brush border of the proximal tubules (black arrows); LEFT: the brush borders are intact in the representative normal image; RIGHT: the brush borders are lost in the kidney biopsy from this patient with suspected vancomycin nephrotoxicity (black arrows) and degenerate cellular casts are seen (open arrow).
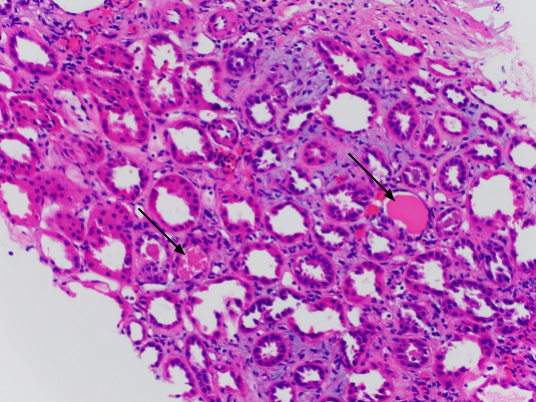
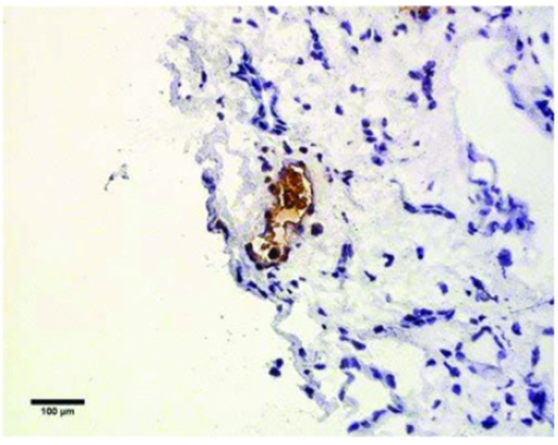
Of the few reports of vancomycin nephrotoxicity in the literature where a biopsy was obtained, acute tubular necrosis and acute interstitial nephritis were the most common histological patterns. Vancomycin-associated kidney damage is likely related to drug-related production of reactive oxidative species, mitochondrial dysfunction, and inflammatory processes leading to cellular apoptosis. Vancomycin-associated cast nephropathy is also another possible mechanism of injury previously demonstrated in kidney biopsies using special immunohistological staining (Figure 5). These staining methods are not widely used or validated, but the hypothesis is that vancomycin-uromodulin complexes may aggregate in the proximal tubules and obstruct Bowman’s space. Vancomycin nephrotoxicity may also demonstrate “lilac casts,” a morphologic mimic of light chain cast nephropathy which stains purple on H&E and Jones methenamine silver.
E. Final Diagnosis
The most likely diagnosis is vancomycin nephrotoxicity. This patient had acute kidney injury with elevated vancomycin levels, without a more likely explanation. The patient had multiple risk factors for vancomycin-nephrotoxicity (obesity, target vancomycin trough >15, vancomycin duration ≥7 days, volume depletion, concomitant nephrotoxin use) despite therapeutic drug monitoring. Kidney biopsy predominantly demonstrated acute tubular necrosis, often seen in vancomycin nephrotoxicity.
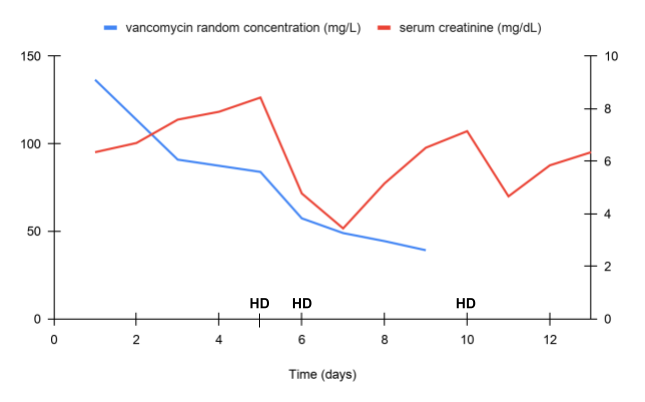
Treatment of vancomycin nephrotoxicity is supportive and includes discontinuing the vancomycin, maintaining euvolemia, and—in some cases of refractory kidney failure—hemodialysis. In most cases, kidney function recovers after discontinuation of vancomycin. Severe cases, however, are exacerbated by oliguria and decreased vancomycin clearance. Vancomycin is a large glycopeptide drug molecule (molecular weight of 1450 Da) that is effectively dialyzable. Vancomycin levels decrease by 30-40% over one dialysis session. Continuous renal replacement therapies also clear vancomycin, to a degree which can be approximated by the effluent flow rate.
In this case, kidney function worsened despite vancomycin cessation, fluid administration, and supportive care. Due to refractory hyperkalemia, the patient was initiated on hemodialysis via a tunneled dialysis catheter (Figure 6). This was a severe case with a protracted recovery period. Fortunately, after 8 weeks her kidney function recovered to baseline, her infection was successfully treated with linezolid as an alternative antibiotic, and she no longer required hemodialysis.
F. Take Home Points
- Vancomycin elimination is dependent on kidney function.
- Elevated vancomycin levels alone do not make the diagnosis of vancomycin nephrotoxicity; other causes of acute kidney injury should be carefully considered.
- Risk factors for vancomycin nephrotoxicity include: obesity, chronic kidney disease, hypovolemia, critical illness, target vancomycin trough >15 mg/L, daily dose >4g, and treatment duration ≥ 7 days.
- Acute tubular necrosis and acute interstitial nephritis are common histologic patterns of vancomycin-associated kidney injury; vancomycin cast nephropathy is a possible mechanism.
- Vancomycin nephrotoxicity usually resolves with cessation of the drug and supportive care; severe cases of vancomycin-associated acute kidney injury may require hemodialysis.
Matthew Sparks, MD, FASN, FAHA, Chi Chu, MD, MAS, Joel M. Topf, MD, FACP, Isabelle, Dominique Tomacruz, MD, Sayna Norouzi, MD, Kartik Kalra, MD, Jamie Willows, Alex Meraz, MD, Tiffany Caza, PhD, MD, Anna Gaddy, MD


