Brandon E. McFarlin, Ph.D. Candidate
Keck School of Medicine of University of Southern California
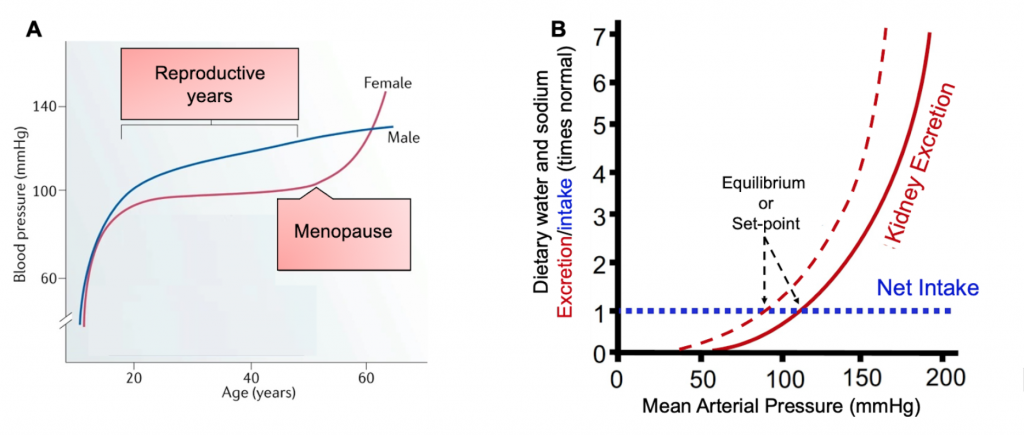
Cardiovascular disease (CVD) is the leading cause of death globally – estimated at 17.9 million lives annually. A major risk factor for CVD is hypertension and in the United States, more than 45% of Americans are estimated to have hypertension. In 2018, hypertension was a primary or contributing cause of death for approximately 500,000 Americans. Hypertension is also a major risk factor of chronic kidney disease (CKD) which puts patients at a four-time greater risk of developing CVD. It is well established that premenopausal females have lower blood pressure (Figure 1A) compared to age-matched males although elevation over time progresses more rapidly in females. The ‘premenopausal female advantage’ in cardiovascular health also manifests in sex-specific differences in CKD prevalence, morbidity, mortality, and treatment outcomes. Sex-specific differences in cardiovascular and kidney function begin to manifest during reproductive years (Figure 1A), which interestingly suggests that females could be optimized for unique challenges faced during these years such as rapidly changing effective circulating volume (ECV) during pregnancy and lactation. Investigations into the mechanisms for these sex-specific differences are complicated and poorly understood. However, it is apparent that there are unique sex-specific differences in blood pressure between males and females that are most likely influenced by many factors. Herein, I will briefly discuss the known roles that sex and sex hormones may play in altering kidney physiology and thus impacting blood pressure.
Pressure natriuresis and effective circulating volume
The kidney-body fluid feedback mechanism, conceptualized by Guyton and colleagues, outlines how pressure natriuresis (generally referring to both diuresis and natriuresis) is a powerful mechanism used by the kidneys for acute and chronic maintenance of ECV. To maintain steady-state conditions, intake must be equal to output (Figure 1B). Imbalances in this equilibrium such as abnormal increases in sodium (Na+) reabsorption or failure of the kidneys to excrete ingested sodium leads to parallel increases in water reabsorption and movement of water from tissues into the blood to correct circulating sodium concentration, ultimately increasing ECV. Increases in ECV elevate arterial pressure, which serves as a homeostatic error signal to correct ECV via pressure natriuresis. Females have a leftward shift in the pressure natriuresis curve (denoted by dotted red line Figure 1B), meaning that females have greater capability to excrete sodium and reduce ECV at similar arterial pressures as males. This effectively establishes their set-point to the left (or at a lower blood pressure) of age-matched males. This innate leftward shift in females is mediated by differences in the kidney’s handling of sodium. According to rodent studies and mathematical modeling of the nephron, females have lower fractional reabsorption in the proximal tubule segment of the nephron due to both lower transporter activity and transport area.
The proximal tubule is responsible for ⅔ of all reabsorption along the nephron and is layered with microvilli to increase transport surface area. In male rats, a key mechanism of pressure natriuresis is the inhibition of proximal tubular transporters utilized in Na+ reabsorption. The Na+/H+ exchanger isoform 3 (NHE3) and Na+/Pi exchanger isoform 2 (NaPi2) redistribute from within the microvilli (active transport) to the base of microvilli (associated with phosphorylation and inactivation) or are internalized into the microvillar cleft, respectively. In female rats however, NHE3 is localized at the base of the microvilli under normal conditions, further contributing to the ability to excrete a Na+ load more rapidly than males. In addition to sex-specific differences in Na+ transport, renal epithelial expression of the anti-hypertensive angiotensin II (Ang II) type II (AT2) receptor, which opposes the prohypertensive AT1 receptor was four-fold greater in females, potentially further contributing to vasodilation and anti-inflammatory properties. Overall, the mediators of pressure natriuresis and the regulation of extracellular fluid volume are complex and multifaceted. More information can be found in reviews on these topics such as Ivy J.R. and Bailey M.A. 2014 and McDonough A.A. et al 2003.
Role of sex hormones
Sex hormones may also contribute to the differences in cardiovascular health between men and women. Many have investigated the influence of sex hormones on blood pressure and these have been extensively reviewed by Colafella, K.M.M. and Denton, K.M., 2018 and Maranon R. and Reckeloff J.F., 2001. A broad consensus can be made that both estrogens and androgens are likely to influence blood pressure in a multitude of ways.
In males, testosterone is the primary androgen and is associated with activation of pro-hypertensive AT1 receptor, sodium retention, greater oxidative stress, a rightward shift in pressure natriuresis relationship (Figure 1B), and a shift in the renin-angiotensin-aldosterone system towards the pressor pathway compared to female counterparts (Table 1).
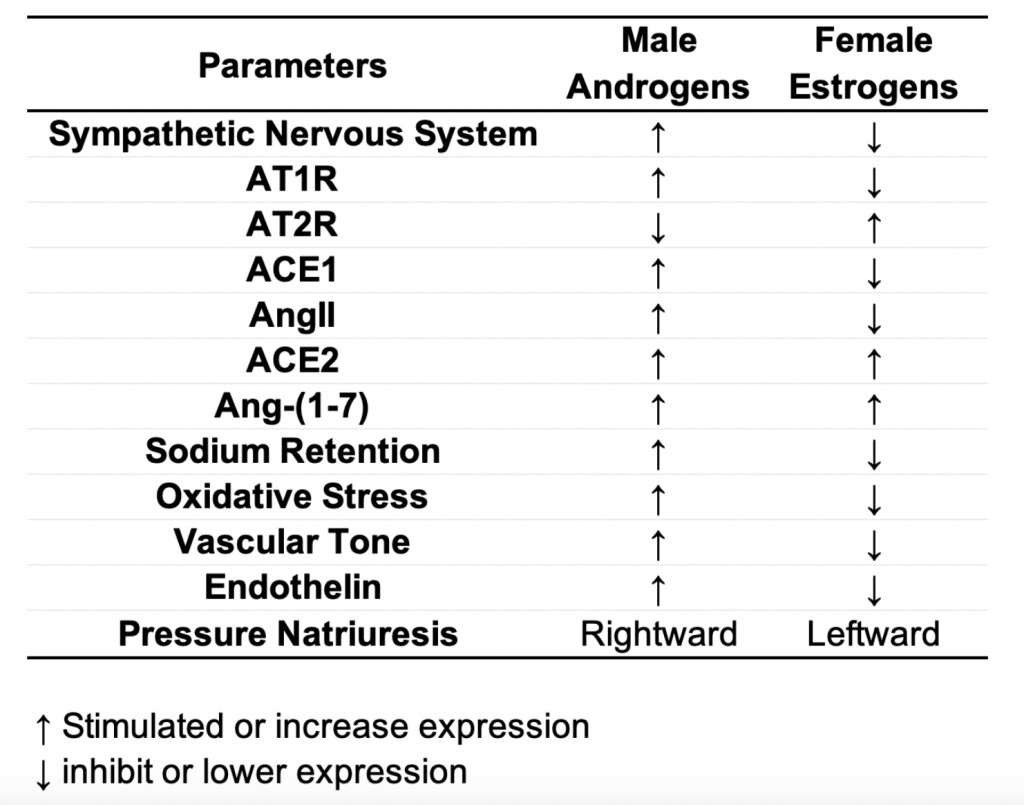
Whereas in females, estrogens have been shown to reduce AT1R expression, enhance AT2 receptor expression, and enhance angiotensin-converting enzyme 2 (ACE2) expression. These mechanisms mediate reduced vascular tone, reduced Ang II, greater Ang-(1-7) in females (breakdown of Ang II), reduced-sodium retention, and a leftward shift in pressure natriuresis curve in females compared to males (Table 1).
However, despite the seemingly dichotomous roles of androgens and estrogens, altering the hormonal balance in either sex to slow the progression of CKD or reduce the risk of CVD is complex and not definitive. Females with elevated testosterone levels (e.g., polycystic ovarian syndrome) or administering exogenous testosterone (5 mg) to ovariectomized spontaneously hypertensive rats (SHR), results in a greater incidence of hypertension and elevations in blood pressure, respectively. However, cases for the beneficial effects of testosterone on glucose and lipid metabolism have also been made. Chronic testosterone deficiency is associated with obesity, which is a risk factor for CVD. Additionally, testosterone levels are lower in males with chronic diseases. While more investigation is necessary to understand the beneficial and detrimental effects of testosterone, these discrepancies suggest that testosterone levels alone do not account for reported sex-specific differences in disease. In contrast, menopause is characterized by a loss of estrogen production and can be accompanied by increases in blood pressure in women. However, hormone replacement therapy often does not significantly reduce blood pressure in postmenopausal women, again suggesting that the loss of estrogens is not the only component involved in mediating hypertension in this group. In addition, blood pressure was not elevated following ovariectomy in SHR, until androgen treatment. Despite numerous sex-specific differences studies investigating AngII induced hypertension have shown no sex differences in vascular response or kidney transporters abundance changes to AngII. Collectively these studies suggest that testosterone levels are necessary for proper male metabolism and blood pressure control, while the female “protection” to cardiovascular disease and even potentially kidney disease is driven by lower androgen levels rather than the presence of estrogen.
Taken together, mechanisms underlying sex-specific differences in blood pressure are complex and need additional investigation. However, these sex-specific differences seem to culminate in lower disease prevalence in females during their reproductive years. This would make sense evolutionarily to optimize females for the unique challenges of pregnancy and lactation. Increases in sodium and water reabsorption can expand ECV 30-50% and increase cardiac output to support fetal development with little to no changes in arterial pressure. Ultimately this leftward shift in pressure natriuresis (Figure 1B), lower proximal tubular reabsorption, and lower blood pressure could be a defense mechanism evolutionarily evolved during reproductive years to protect females and fetal development that is lost after menopause.
Reviewed by: Elinor Mannon, Kelly Hyndman, PhD, Matthew Sparks, MD, FASN, FAHA



Well done, Brandon! You have hit the nail on the head. It is the effective circulating volume that is regulated – not blood pressure. Changes in BP reflect homeostatic adjustment driven by BP to normalize ECV. Whether these pressure driven responses are restricted to kidney alone we can’t be certain, but the kidney is certainly important. Alicia