To stain, or not to stain, that is the question.
Different illumination techniques are available to visualize the formed elements in the urinary sediment. The two most commonly used are brightfield and phase contrast. Brightfield illumination provides high resolution images only with naturally pigmented specimens or with use of a stain. Without pigmentation or staining, items with a low refractive index, such as hyaline cast matrix and cellular contents, are almost invisible, with insufficient contrast to properly identify structures. Phase contrast on the other hand enables visualization of elements with a low refractive index by augmenting contrast. This effect is best with unstained thinly prepared specimens.
Staining may alter or obscure the color characteristics of elements in the urinary sediment and, under phase contrast, may cause “phase artifacts” which significantly degrade image quality. Staining can also result in formation of “pseudocasts” by facilitating aggregation of amorphous debris and cells.
Therefore, to answer our original question “To stain, or not to stain?”: Both! When possible, prepare two samples – one stained, and the other unstained.
Brightfield illumination is most effective when viewing a stained specimen whereas phase contrast is best when viewing a thinly prepared unstained specimen.
Sternheimer-Malbin (SM) Stain
The best all around stain for urine microscopy is the Sternheimer-Malbin (SM) stain. This is a supra vital stain composed of a stabilized mixture of crystal violet and safranin which greatly facilitates identification of WBC’s, epithelial cells, and casts. It is quite simple to use and requires no fixation or special steps: during the usual specimen preparation process, after centrifugation and pouring off the supernatant, resuspend the sediment and add one drop of stain. Mix gently and allow 1-2 minutes for uptake of stain before preparing the slide.
The rendered color and degree of staining depends upon duration of exposure to stain and the urine pH. Selective permeability of viable cells allows them to resist stain uptake whereas nonviable or damaged cells are readily stainable.
Patterns of staining with Sternheimer-Malbin Stain:
- Hyaline cast protein matrix appears pale pink to purple
- Granular casts appearance is variable ranging from dark pink, to magenta or purple
- Waxy casts appear pale pink to dark purple
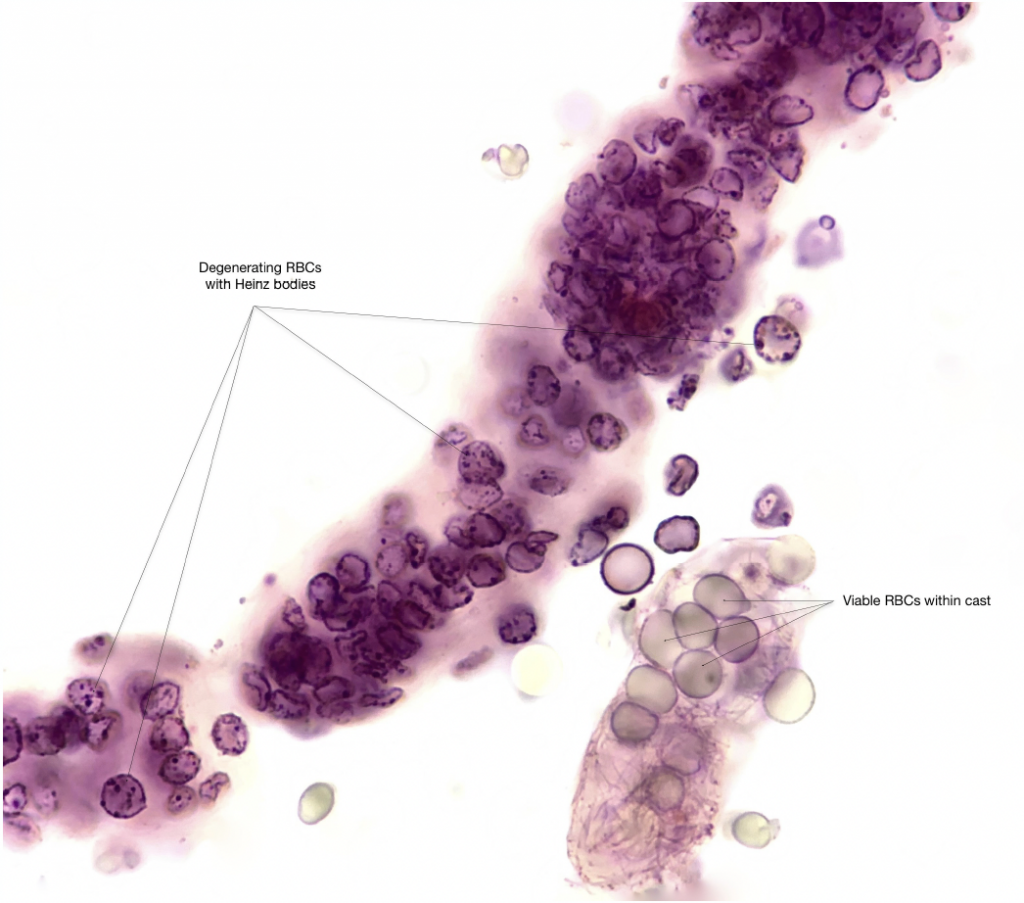
- Viable RBC’s appear unstained. Older or nonviable cells may appear pale pink to magenta
- There are two different WBC staining patterns observed with use of the SM stain. Dark staining neutrophils are characterized by translucent or granular cytoplasm and avid magenta or red staining of the segmented nucleus. These cells are older and no longer viable. Pale staining neutrophils have pale indistinct blue staining of the cytoplasm and nucleus. These cells remain viable and may have visible cytoplasmic granule movement (“glitter cells”).
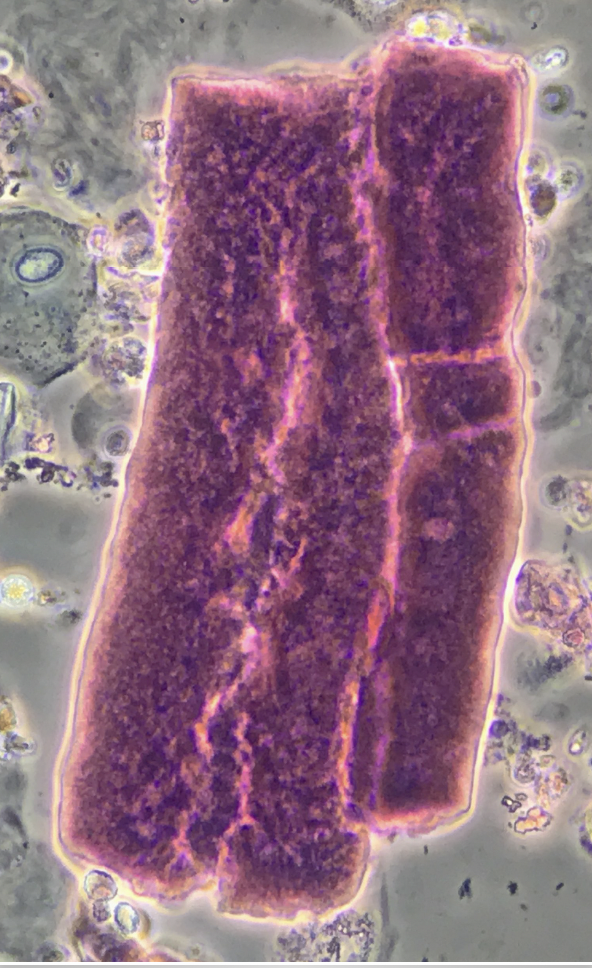
Waxy cast – phase contrast, with SM stain ![]()
Granular casts – brightfield, with SM stain 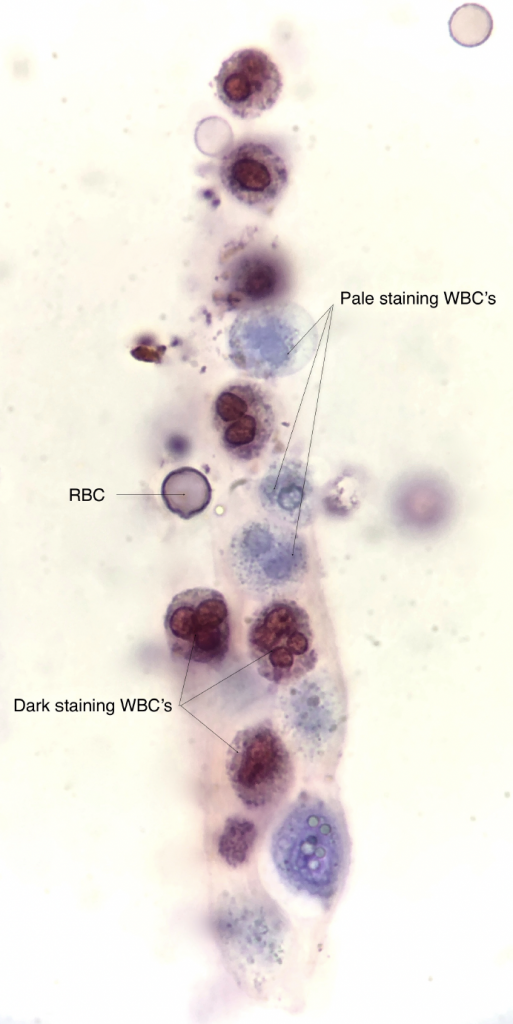
Dark staining WBC’s are older, nonviable cells whereas the pale staining WBC’s are younger and remain viable — brightfield, with SM stain ![]()
Old, degenerating RBC’s are readily stained whereas younger, viable RBC’s appear relatively unstained — brightfield, with SM stain
Sudan III Stain
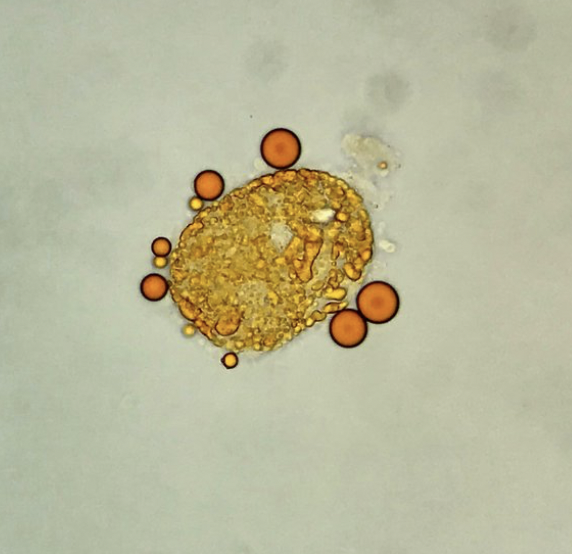
Sudan III is a lipophilic stain useful for the identification of lipids (neutral fats and triglycerides) in the urine, as an adjunct to polarization, or when polarized microscopy is not available. After usual sediment preparation add 3-5 drops of Sudan III and mix gently. Allow 15 minutes for stain uptake, then place a drop of this mixture on a slide with a cover glass. Lipid droplets (either as free droplets or incorporated in oval fat bodies or casts) appear yellow to orange/red.
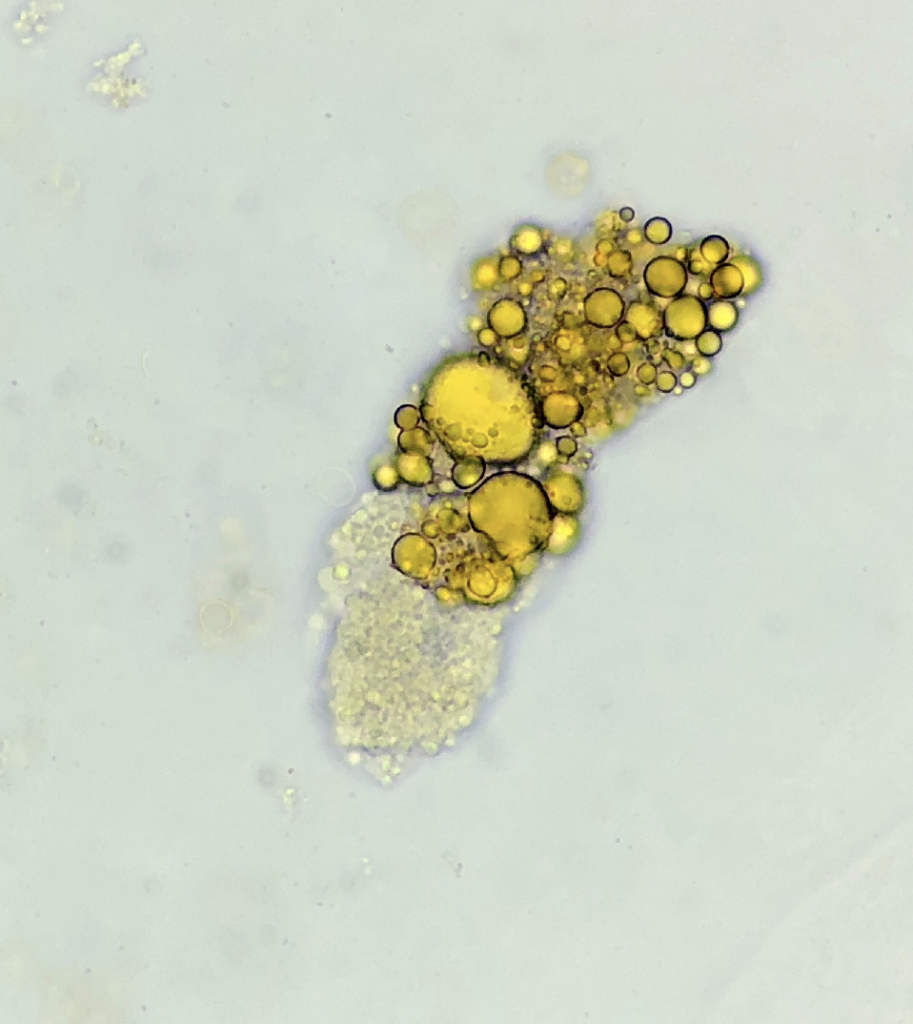
Lipid cast – Sudan III stain ![]()
Oval fat body and free lipid droplets – Sudan III stain
Post by: Jay Seltzer, MD

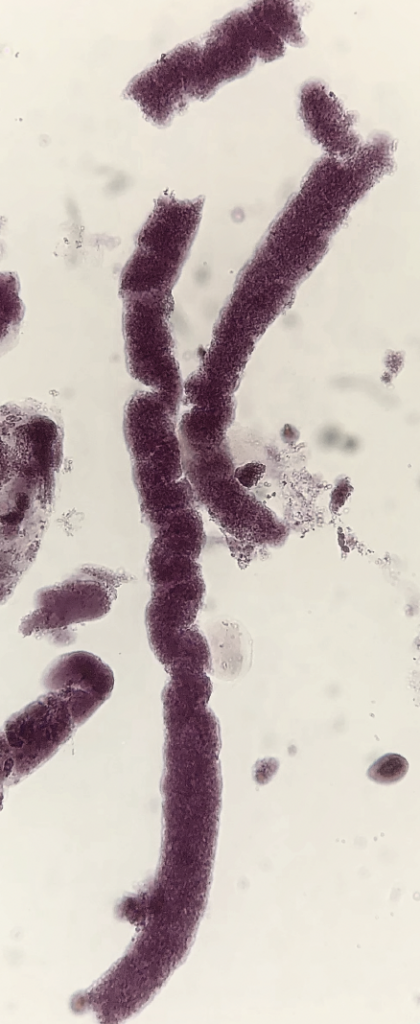


Since the SM stain contains crystal violet and safranin, could it be used for gram staining?