Welcome to the 26th case of the Skeleton Key Group, a team of 50-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network. These are actual cases (without patient identifying information) that intrigued the treating physician.
This month we bring in a pediatrics case with long standing hyponatremia. Can you guess what’s going on?
Written by: Archana Vajjala, S. Sudha Mannemuddhu, Shweta Shah
Visual Abstract by: Shweta Shah
A. The Stem
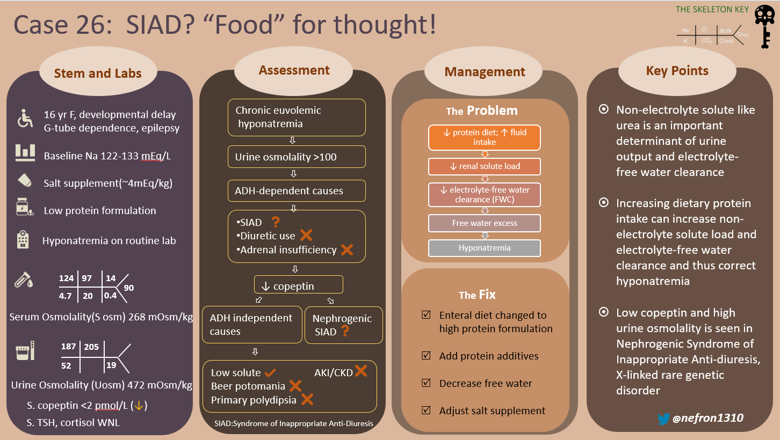
A 15-year-old adolescent female with agenesis of the corpus callosum, global developmental delay, epilepsy, spastic quadriplegia, and gastrostomy tube (G tube) dependence, presented to the ER after a routine gastroenterology visit, due to a serum sodium of 124 mEq/L. The parents have not noticed any change in the child’s behavior. Her sodium a month ago was 131 mEq/L. She has a history of multiple episodes of hyponatremia (Na 124–133 mEq/L) over the previous 2 years, requiring multiple admissions and ER visits. This was ascribed to syndrome of inappropriate antidiuresis (SIAD) of unclear etiology.
At the time of this ER visit, she was taking 9 g of table salt (NaCl) and 6 g of Lite Salt™ (a salt substitute containing 50% NaCl and 50% KCl) daily.
Physical Exam:
Temp 98.8 °F; HR: 76 bpm; BP 119/90 mm Hg; Sat 99% on room air.
BMI 17.1 kg/m2; Weight 34.1 kg
The examination is unchanged from baseline. She is microcephalic, non-verbal, non-ambulatory (wheel-chair dependent), with spasticity and contractures of all limbs. Her cardiorespiratory status is stable. There are no signs of infection.
She wears incontinence pads. Her mother does not report any change in urine output.
Nephrology is consulted for evaluation and management of hyponatremia.
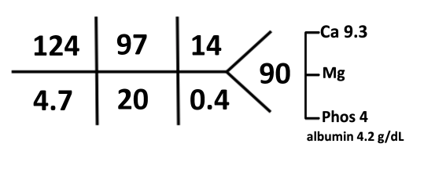
B. The Labs
C. The Workup
What is the cause of hyponatremia?
Here is a figure for the initial approach to hyponatremia (Figure 2).
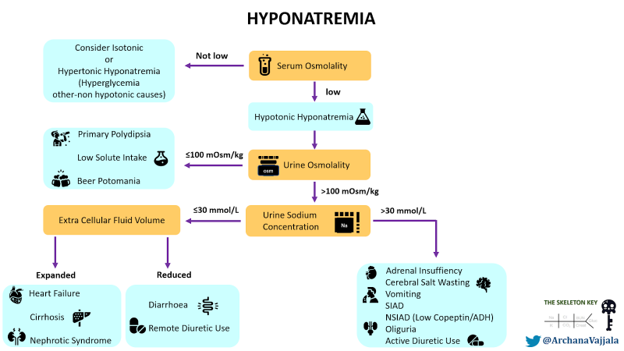
The first step in evaluation of a low sodium level is to confirm true hyponatremia by measuring serum osmolality. This patient has true euvolemic hypoosmolar hyponatremia with serum osmolality 268 mOsm/kg). Since we cannot confirm the duration of hyponatremia to be <48 hours, we consider and treat this as chronic hyponatremia.
The next step in evaluation is to determine if hyponatremia is being driven by antidiuretic hormone (ADH) activity in an ADH-dependent process, or if hyponatremia is occurring without ADH activity (an ADH-independent process). Secretion of ADH, also known as arginine vasopressin (AVP), leads to water reabsorption in the distal nephron increasing the total body water (TBW), diluting plasma sodium. High ADH causes an increase in urine osmolality (concentrated urine), while low ADH results in low urine osmolality (dilute urine).
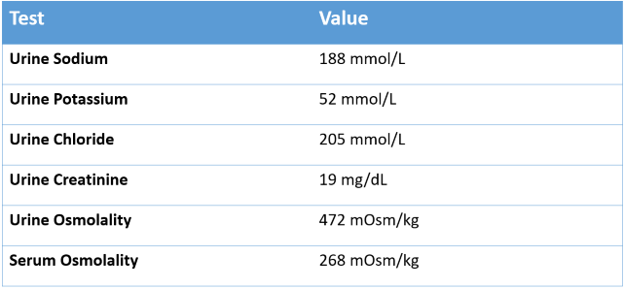
Our patient had a urine osmolality of 472 mOsm/kg with 15 g salt supplementation each day, and remained in a relatively fixed range, suggesting inappropriate ADH activity.
Given the long standing hyponatremia [S.Na 124-134 mEq/L] with elevated urine osmolality [400-600 mOsm/kg] and urine sodium >100 mmol/L, she was diagnosed with syndrome of inappropriate antidiuresis (SIAD), but repeated previous evaluations failed to identify a specific cause of SIAD.
More Data:
She was strictly tube-fed with an enteral formula; a specific fluid restriction was not recommended at that time. One of the goals of therapy is to increase water excretion by increasing solute load to the kidneys. In some forms of SIAD, antidiuretic hormone (ADH) no longer regulates water excretion independently and so water excretion becomes a dependent variable (depends on the solute intake and UOsm). To increase solute load, she started taking 4.5 g of table salt (70 mEq of Na) daily which was eventually increased to 9 g along with another 6 g of salt Lite (50% NaCl and 50% KCl).
Despite salt supplementation her hyponatremia remained, so we took a deep dive into her diet in order to examine her daily water and solute intake. The amount of solute generated/consumed each day is the amount that must be excreted by the kidneys and is known as the renal solute load.
She is G-tube dependent. She was on a low protein, egg-flavored formula (meal supplement) called Real Food Blends™, kefir milk and free water flushes as shown in the table. Additionally, she received 9 g of table salt with 6 g of Lite Salt™. She was on a lower protein meal supplement due to parental preference and unaware that different flavors have different protein composition. Osmolality and free water in her daily feeds are as shown in Table 1.
Patient is incontinent and exact urine output was not known. Patient had a relatively fixed urine osmolality despite her low solute load, suggesting inappropriate ADH effect. As mentioned above, the goal of therapy was then directed to increasing solute load to increase urine volume.
Let’s discuss renal solute load (RSL) calculation here as it was very relevant to how we treated this patient.
The RSL is the total osmoles that need to be excreted by the kidneys per day. The major sources of osmoles are nitrogenous wastes (from dietary protein) and minerals (from dietary electrolytes including sodium, potassium, chloride, phosphorus, and calcium).
Under normal conditions, RSL (mOsm) = (gm of protein intake x 5.7) + (mOsm of Na+K+Cl)
In children, each gram of protein yields an estimated 4 mOsm instead of 5.7 in adults due to growing needs.
1 mEq of Sodium, potassium, and chloride each yields 1 mOsm
Urine output depends on renal solute load and urine osmolality which varies from 50-1200 mOsm/kg depending on ADH secretion. The minimum volume of urine (L) needed to excrete the renal solute load (RSL) with maximum urine osmolality of 1200 mOsm would be RSL (mOsm) /1200 mOsm/L.
If a patient has a low RSL and a fixed urine concentration, the urine output will be decreased.
The renal solute load for our patient:
Calculated renal solute load from the formula and salt supplement would be:
RSL (mOsm) = [Protein in gm x 4 + (Na+K+Cl in mEq from feeds)] + [Na+K+Cl in mEq from salt]
= [27 x 4 +(42 mEq)] +[306+183 mEq]
= 150+489
= 639 mOsm
estimated urine output ~ 1350 ml, based on urine osmolality of 472 mOsm/kg. Exact urine output was not known for our patient.
Any water intake in excess of urine output would be retained and contribute to hyponatremia. Urine volume is constituted by free water clearance and solute clearance. The following equation explains how solute clearance can affect free water clearance and impact plasma sodium.
Total Urine Volume = Free water clearance + Solute Clearance
Free water clearance can be calculate using the following equation:
Free water clearance Cwater = Urine Volume(V) – Solute Clearance
Cwater= V – (Uosm*V/Posm)
Cwater= V(1 – Uosm/Posm)
Where V is urine volume
Negative (-) free water clearance suggests net free water retention while positive (+) free water clearance suggests net free water excretion.
Since no cause for SIAD was identified, a copeptin level was obtained: Copeptin <2 pmol/L
What is Copeptin?
Copeptin is the part of the ADH prohormone (Figure 3) from the posterior pituitary gland. It is secreted together with ADH into the circulation in a 1:1 ratio and because of its stability compared to ADH, can be measured as a surrogate marker of ADH.
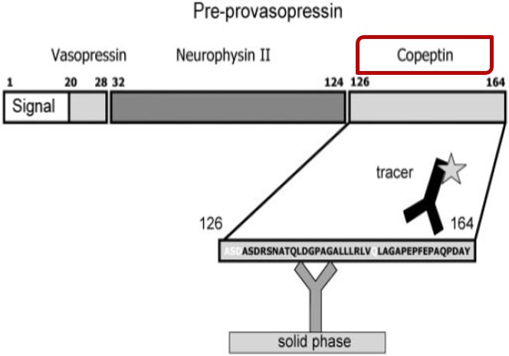
A new copeptin‐based diagnostic workflow for the differential diagnosis of polyuria‐polydipsia syndrome incorporates copeptin levels in exploring the different etiologies of hyponatremia (Figure 4).
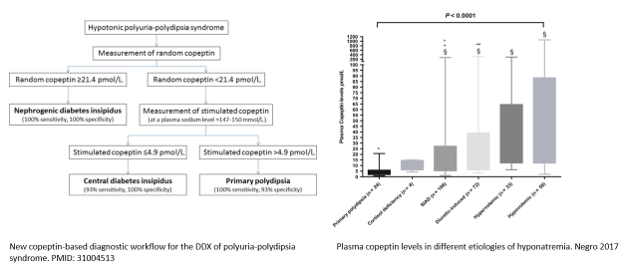
D. Final Diagnosis
Low solute intake combined with high fluid intake can cause hyponatremia, commonly seen in patients with low protein diets. However, this patient has elevated urine osmolality (~ 500 mOsm/kg) when copeptin is undetectable, pointing toward the possibility of Nephrogenic Syndrome of Inappropriate Anti Diuresis (NSIAD).
What is NSIAD- Nephrogenic Syndrome of Inappropriate Antidiuresis?
NSIAD is a rare X-linked (recessive) genetic disorder due to a gain of function mutation in vasopressin 2 receptor, V2R gene (Xq28), coined in 2005, however it was originally reported vaguely in 1957. This could be conceptualized as the opposite of X-linked Nephrogenic Diabetes Insipidus. The clinical presentation is consistent with SIAD but with undetectable levels of ADH or copeptin. There are various missense mutations described in V2R leading to NSIAD. The frequency of NSIAD is not known, but could be underestimated. As many as 10 to 20% of patients with SIAD have ADH/copeptin levels at or below the limits of detection by radioimmunoassay and some of them at least could have NSIAD. According to Orphanet, 21 cases of hyponatremia due to NSIAD having been reported to date, the majority of them coming from 5 families, and 3 of them were females. Due to variable expression and variable phenotype of NSIAD both in male and female individuals, it might go unrecognized for years. Patients can present anywhere from infancy to late childhood or adulthood. Although it is a recessive disorder, X-inactivation/ lyonization may cause an NSIAD phenotype to present in females. Mutant V2R induces cAMP at least 4–7.5-fold compared with the wild-type V2R, which could explain the very low/ undetectable levels of ADH or copeptin (Figure 5).
Typical features include hyponatremia, seizures and the lack of urinary dilution in the presence of hypoosmolality. Usually hyponatremia in these cases is asymptomatic until severe. In patients with euvolemic hypotonic hyponatremia, increased urinary osmolality (> 100 mosm/kg), increased urinary sodium (> 30 mmol/l), normal thyroid, adrenal, cardiac and renal function and the absence of diuretics with low ADH/copeptin levels, and in patients with unexplained intermittent hyponatremia, NSIAD should be considered. Definitive diagnosis is by genetic testing.
Fluid restriction and oral urea are treatment options. Fluid restriction, though effective, may not be entirely feasible in infancy. Furosemide and ADH are being studied as possible treatment options.
Vaptans (vasopressin competitive antagonists) will likely be ineffective, as there is no ADH to antagonize in NSIAD.
Figure 4 is an infographic describing and differentiating various forms of ADH and its receptor related dysnatremias.
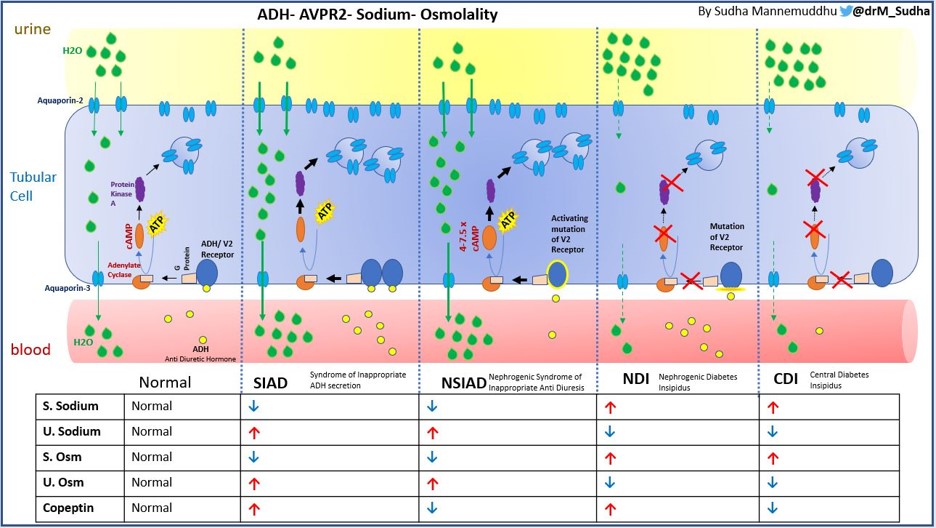
E. The Management
We managed this patient’s hyponatremia by increasing protein intake, thus increasing solute load. Suboptimal nutrition in this adolescent has led to decreased solute load from protein metabolism (which produces urea, a major osmole in the urine). The reduced solute load limits the ability of the kidney to excrete electrolyte free water. As illustrated in the equation above, urine volume is solute load divided by urine concentration. By increasing protein intake, renal solute load increases significantly, thus with fixed urine osmolality, the volume of urine excreted could be increased and thus electrolyte free water clearance.
Over the course of time changes were made by nutritionist as follows-
Real Food Blends (Turkey, chicken, or salmon) 480 mL, Kefir 360 mL, and Beneprotein (4 scoops/ day) to provide a total of 925 kcal (~27 kcal/kg), 68 g protein (2g/kg), and ~1445ml free water per day. Osmoles and free water in her daily feeds is shown in Table 2.
New renal solute load with increased protein intake and 6 g table salt and 3g of salt Lite (KCl and NaCl)
= 626 mOsm
Labs 3 months after the presentation:
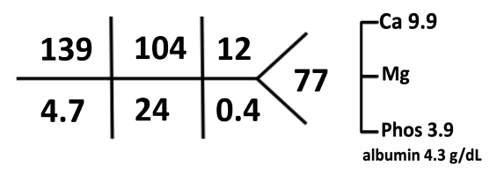
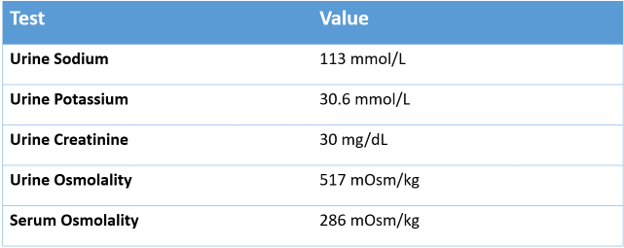
In this patient, if you observe carefully, the total osmoles generated by the formula and salt combination before and after are equal. However, protein content was doubled and salt halved.
The improvement is best visualized by moving from free water clearance to electrolyte free water clearance, which governs tonicity. The equation for electrolyte free water clearance is:
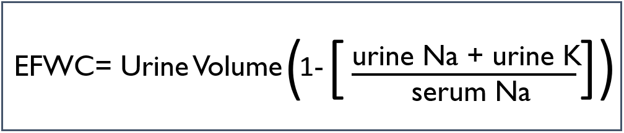
The ratio of urine cations to serum cations is called the Furst ratio, and a ratio over one will result in a negative electrolyte free water clearance which means that every time a patient makes urine they are actually adding electrolyte free water to the body. The Furst ratio on presentation was
(187 + 42) / 124 = 1.85
The Furst ratio after the intervention was much improved at
(113+30) /139 = 1.02
Essentially the therapy resulted in the patient making isotonic urine so that modest fluid restriction was able to correct the sodium.
In NSIAD, Urine osmolality is fixed given that mutated V2R is always active.
Free water clearance includes electrolyte free water clearance and non-electrolyte free water clearance which is primarily contributed by urea excretion. We increased urea excretion by a high protein diet and thereby total free water clearance. She was followed for at least 1.5 years after switching the formula and there were no hospitalizations or ER visits during that period and her serum sodium remained >135 mEq/L.
F. The Take Home Message:
- Non-electrolyte solute excretion, like that of urea, is an important determinant of free water clearance. This is low in conditions of low(er) protein intake and important etiological and therapeutic consideration in hyponatremia.
- Increasing the solute load increases the electrolyte free water excretion thus increasing serum sodium if total fluid intake remains the same
- In patients with hyponatremia without any apparent cause or SIAD with low copeptin levels, Nephrogenic SIAD (NSIAD) should be considered.
Reviewed by Joel Topf, Chi Chu, Dominique Tomacruz, Dilushi Wijayaratne, Raad Chaudary, Jefferson Triozzi, Matt Sparks