Brian Rifkin, MD
Hattiesburg Clinic
Acknowledgements: This post is part of a collaboration between The Renal Fellow Network and the American Society of Diagnostic and Interventional Nephrology (ASDIN), whose mission is to provide excellence in dialysis access care to improve outcomes for patients with kidney disease. For more information about the ASDIN mission or membership, click here. Special thanks to the ASDIN Fellows Education Committee for their support on this project.

“No sheath shall hold what finds its home in flesh” -Ukrainian Proverb
Introduction: Data from the United States Renal Data System (USRDS) demonstrate that the percentage of patients who initiated hemodialysis using central venous catheters (CVCs), for at least 3 months of hemodialysis (HD), actually increased in 2020 to 11.9%. Additionally, the percentage of patients initiating HD with a catheter also increased in 2020 to 71.2%, and a majority of these patients were still using CVCs for dialysis access 90 days later. CVCs are convenient because they can be used immediately after placement, but they have a much higher incidence of dysfunction and infection than dialysis grafts or fistulas. One unique problem for catheters is the formation of fibrin sheaths. A fibrin sheath (imagine a structure similar to a sword’s sheath) can completely envelop a CVC and obstruct or impede flow. The sheath covers the inlet and outlet holes of the hemodialysis catheter acting as a one-way valve. Even partial sheaths can prevent the high flow rates required for satisfactory hemodialysis. Fibrin sheath formation can be seen in up to 76% of CVCs at some point in their use. In experimental studies, fibrin sheath formation starts as early as 24 hours after insertion of the catheter, with encasement of its entire length occurring within 5 to 7 days in some cases. KDOQI provides limited guidance on fibrin sheaths (see guideline 26.3 above), as there are currently very few evidence-based interventions. Unfortunately, we have not learned very much since Dr. Nathan Hellman opined about fibrin sheaths and catheter dysfunction in a very early Renal Fellow Network post.
How are sheaths formed and what is their composition? What are the best strategies for disruption of sheaths? And finally, what strategies can be used to prevent sheath recurrence?
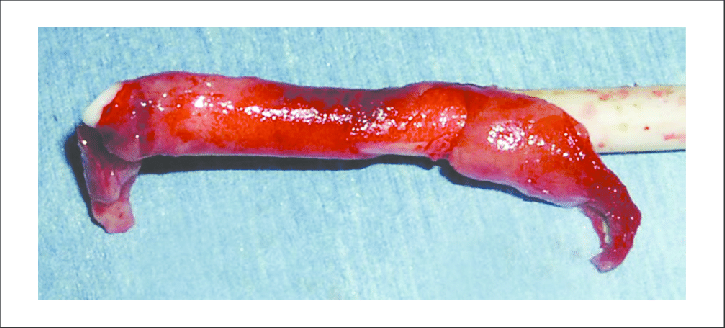
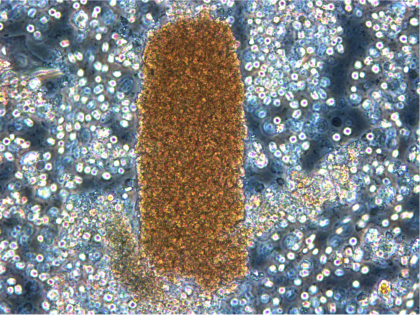
Fibrin Sheath as seen on catheter removed from the body
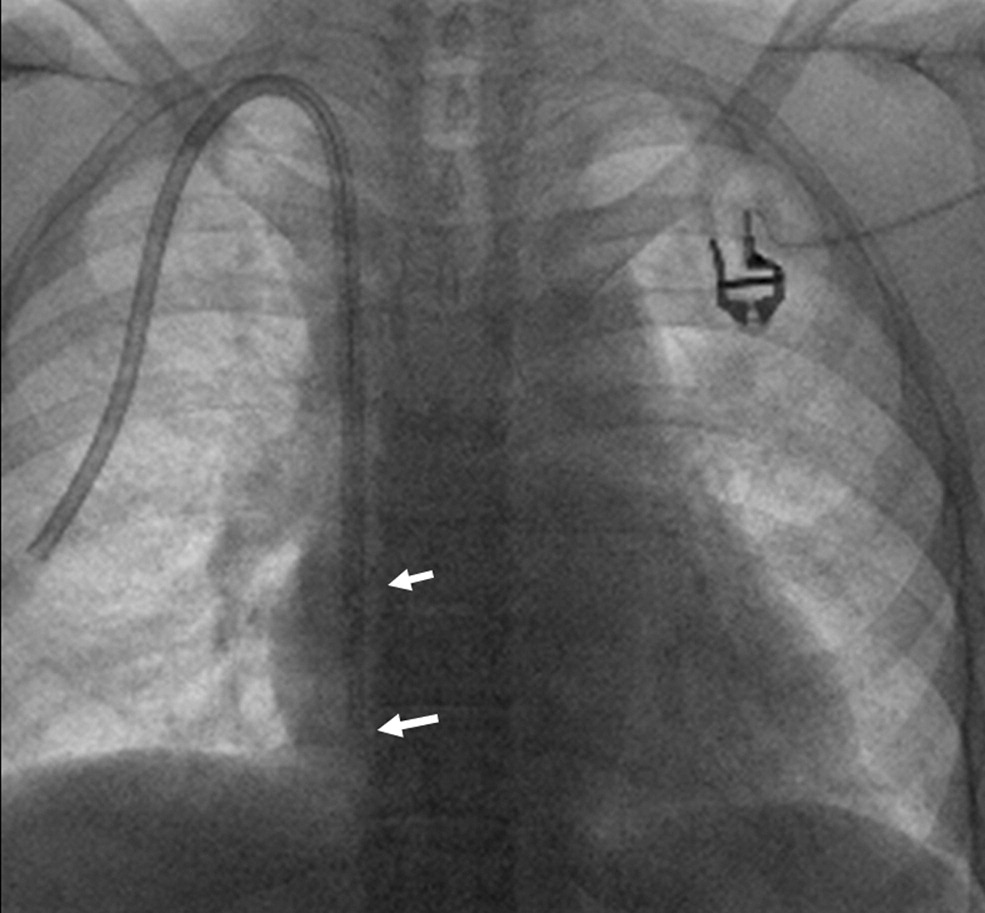
Fibrin sheath (white arrows) following catheter pullback to upper sternum and contrast injection
Sheath Formation: Fibrin sheaths are comprised of a heterogeneous composition of cells, thrombi, and proteinaceous material. When a patient is sent for evaluation of a dysfunctional catheter, a sheath can be easily recognized by doing a venogram after pulling back the existing catheter to the level of the clavicle, injecting contrast, and observing if contrast remains concentrated along the previous track of the catheter. Soujanen et al found that fibrin sheaths, obtained from stripping dialysis catheters, had several different histologic patterns with varying amounts of eosinophilic material, inflammatory cells, organized fibrous tissue, endothelial proliferation all superimposed on thrombi. Xiang et al reported the sleeves around CVCs in a rat model were composed of cellular-collagen tissue covered by endothelium formed by smooth muscle cells migrating from the injured vein wall onto pericatheter thrombi. In extreme cases, the catheter can firmly adhere to the surrounding tissue, becoming tethered to the vessel wall, making removal significantly more complex. Thus, the terms “fibrin sleeve” or “fibrin sheath” inadequately recognize the complex composition of the diverse materials that are incorporated into a sheath.
Anticoagulation/antiplatelet treatment: The findings in the above studies looking at the composition and environment for sheath propagation suggested that if pericatheter thrombi could be mitigated there might be a lower likelihood of sheath formation. Placing patients on anticoagulation or antiplatelet agents to prevent clot formation, an early scaffolding for sleeve genesis, therefore might decrease the risk for catheter dysfunction.
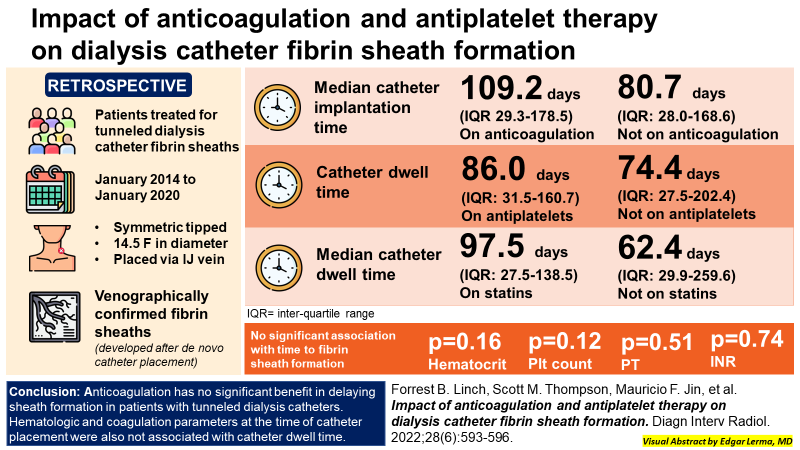
Unfortunately, to date there is limited data on the utility of anticoagulation for the prevention of fibrin sheath development. In a prospective randomized control study by Mokrzycki et al in 2001, the potential benefit of minidose (1 mg) warfarin versus placebo for late malfunction of tunneled dialysis catheters was studied. The authors reported fewer fibrin sheath-stripping procedures in the warfarin group (41 patients) than in the placebo group (44 patients), though the difference was not statistically significant in this small study. Regrettably, there were no prior studies that investigated therapeutic dose anticoagulation for the specific prevention of fibrin sheaths. In 2015 a prospective trial of 25 cancer patients utilizing CVCs with fibrin sheaths detected by ultrasound at 6 months after catheter placement, were placed on low-molecular-weight heparin (LMWH). Thirteen of these 25 patients had no detectable fibrin sheath by ultrasound at 6-month follow-up, while persistent sheaths were detected in 11 patients, and 1 patient developed subocclusive deep vein thrombosis. Once again, the study was small and no patient required catheter exchange for dysfunction. The study concluded fibrin sheath resolution occurred independently of LMWH therapy. Finally in 2022, Linch et al did a single center retrospective review of the impact of anticoagulation and antiplatelet therapy on dialysis catheter sheath formation. Patients on anticoagulation had a longer median catheter implantation time of 109.2 days compared to 80.7 days among patients not on anticoagulation. Catheter dwell time among patients taking antiplatelet therapy was 86.0 days versus 74.4 days for patients not on antiplatelet medication. Patients taking statins versus those not taking statins had median catheter dwell times of 97.5 days and 62.4 days, respectively. Time to fibrin sheath formation was not significantly associated with hematocrit, platelet count, or PT/INR. Ultimately, these studies did not consistently show a beneficial effect of anticoagulation and antiplatelet medications for the prevention of catheter thrombosis or sheath formation. Larger randomized control studies should be done and need to take into consideration the risk and cost of serious hemorrhage of various anti-thrombotic agents versus recurrent catheter interventions.
Sheath disruption: Fibrin sheath formation around long-term hemodialysis catheters are a common cause of failed dialysis access. Techniques for sheath disruption include pharmacologic thrombolysis, mechanical disruption using guide wires with or without balloon angioplasty and snare ”stripping” techniques. Availability, reimbursement and interventionist training may all play a part in the decision making process concerning which methods are employed.
Thrombolytic agents (TLA) can be directly instilled into catheters, in many cases while the patient is still present at the dialysis center. The TLAs evaluated to date include urokinase, streptokinase and the recombinant tissue type-plasminogen activators (tPA) alteplase, reteplase, and tenecteplase. Pharmacological therapy typically involves installation of urokinase (5,000 units or above) or tissue plasminogen activator (2.5 mg in 50 mls normal saline over 3 hours) to lyse the thrombus. The advantage of tPA over urokinase and streptokinase is that tPA is fibrin-specific, and tPA bound to fibrin has greater affinity and enzymatic activity on plasminogen. While efficacious, these agents still have the potential to induce systemic fibrin breakdown and life threatening hemorrhage. However, most studies examining safety profiles of thrombolysis, either by dwell or infusion, observed very low serious hemorrhage rates. A systematic review and meta-analysis in 2013 identified Hemmelgarn 2011 as the only randomized controlled trial of tPA for the prevention of catheter malfunction. That study concluded the use of tPA, instead of heparin as a catheter locking solution for CVCs, significantly reduced the incidence of catheter malfunction and bacteremia. A Cochran Review in 2017 was unable to create a summary of findings of TLAs in catheter sheath formation due to the quality of the body of evidence, a number of study biases and the wide variety of interventions used. TLAs deserve further examination in CVC sheath prevention.
Next, wire and balloon disruption of sheaths is another commonly used technique. First, a wire itself may be used to puncture or snare the sheath. During a catheter exchange procedure a wire is often passed through the dysfunctional catheter in order to maintain a pathway for a new catheter to be directed into the superior vena cava (SVC). Inflating an angioplasty balloon within the dialysis catheter, over an intraluminal wire, for fibrin sheath disruption prior to removal/exchange is possible and well described in the literature (tunneled line intraluminal plasty, TuLIP). Similarly, a balloon may be placed over the wire after the existing catheter has been removed from the body, but prior to reinsertion of a new catheter. In this technique an inflated angioplasty balloon may be inflated in the SVC and briskly brought back and forth to the level of the clavicle, disrupting the sheath or dislodging it from the vessel wall all together. These techniques are not without complications and may include venous rupture and blood vessel irritation or damage which may be linked to neointimal hyperplasia. Ni et al performed a retrospective review of 209 percutaneous transluminal angioplasty balloon disruption and 1304 over-the-wire catheter exchange procedures performed in 753 patients. Of the 753 patients in the study, 127 patients underwent balloon disruption of fibrin sheath and 626 had catheter exchanges. Within the balloon disruption group, 18 (14.2%) of 127 patients subsequently developed central venous stenosis, compared with 44 (7.0%) of 626 in the catheter exchange group (P < 0.01). Time to central venous stenosis development was approximately 3 years in both groups and not significantly different. Finally, once the fibrin is “freed” from the catheter or vessel wall, it typically flows towards the pulmonary arteries. There are infrequent reports of clinical pulmonary emboli as well as case reports of septic pulmonary emboli directly related to fibrin sheath dislodgement.
Snares are an additional tool interventionalists can deploy to capture a sheath from an existing CVC. If the existing permacath tunnel in the neck is used, the dysfunction catheter must first be removed and a new catheter replaced after the sheath is snared. The femoral technique requires puncture of the common femoral vein, with sheath placement for access, and may cause discomfort for the patient and increased potential for intravascular complications and bleeding. A wire snare can be threaded up the inferior vena cava, through the heart, to the existing catheter in the SVC. The snare is then placed around the catheter and gently drawn down the length of the catheter in order to strip off the sheath. The snared sheath may then be removed from the body or released into the bloodstream. The femoral technique has the benefit of allowing the existing catheter to not be removed or exchanged, which may help preserve future venous access sites.
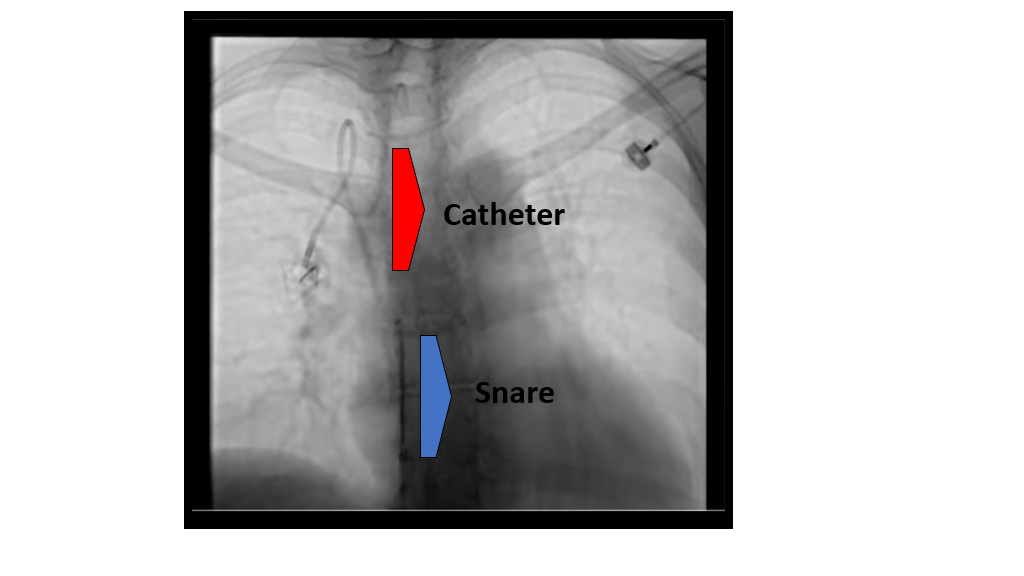
Video: snare “stripping” of fibrin sheath, femoral approach
Conclusion: Although we might try to limit their use, dialysis catheters continue to be the main vascular access used at dialysis initiation. Catheter dysfunction is common and may be due to catheter length, kinking, thrombosis or sheath formation. Newer catheter designs and materials have yet to solve the latter two problems. Disruption of a fibrin sleeve can be accomplished via medications or by minimally invasive mechanical means. Further research is needed to determine the ideal method to restore catheter function while minimizing cost, risk and patient discomfort. Ideally, fibrin sheath disruption should be accomplished quickly and easily with minimal delays in resuming dialysis therapy. Finally, strategies to prevent fibrin sheath formation require larger randomized control studies to directly compare techniques and examine newer anticoagulants and/or antiplatelet medications. This is an area of dialysis access management that currently has very few evidence based therapies, and is overdue for further evaluation.
Reviewed by:
Matt Sparks, Margaret DeOliveira