Post by: Namrata Parikh.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of autoimmune disorders whose hallmark is inflammation of small blood vessels throughout the body, producing a myriad of manifestations. AAV had once been considered to be a rare disease with a reported prevalence of 48-184 cases per million persons. However,it is now believed to be much more common and the current reported prevalence is around 300-421 cases per million persons.
Included within the spectrum of AAV are three main disorders, granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic GPA (EGPA). All three produce characteristic clinical manifestations. In the kidney, small vessel involvement gives rise to a characteristic pauci-immune glomerulonephritis with leukocytoclastic vasculitis, which manifests as proteinuria, microscopic hematuria and/or diminished kidney function.
The pathophysiology of AAV is a loss of immune tolerance (due to various genetic and environmental factors), which leads to production of auto-reactive antibodies, namely, anti neutrophil cytoplasmic antibodies (ANCAs). ANCAs are directed against specific antigens located in the cytoplasm of neutrophils, which form the body’s first line of defence against external stresses. The most well characterized antigens against which ANCAs are formed are myeloperoxidase (MPO) and proteinase 3 (PR3). Other antigens include lysosome associated membrane protein 2 (LAMP2), complementary PR3 (cPR3) peptides, moesin, plasminogen, peroxidasin, and pentraxin. Subsequent infection and inflammation leads to ‘priming’ of the neutrophils and expression of these antigens on the surface, where interaction with preformed ANCAs leads to neutrophil activation, precipitating endothelial damage and inflammation and damage of the blood vessels termed vasculitis. More details regarding pathogenesis can be found here.
The kidneys are involved in up to 70% of patients with GPA and in almost 100% of patients with MPA. Moreover, involvement of the kidneys is associated with a worse outcome in these patients. Hence, it is essential to have a treatment approach which can effectively control the disease process in the kidneys.
Initially, cyclophosphamide was the cornerstone of AAV treatment. See our previous timeline here. However, in recent years, rituximab has emerged as an additional therapeutic option with a better safety profile. Rituximab acts on CD20 receptors on peripheral B cells and inhibits the immune mediated destruction by several mechanisms:
- Complement activation following binding to CD20 leading to cell lysis, or complement-dependent cytotoxicity.
- Antibody dependent cell-mediated cytotoxicity via natural killer cells
- Caspase activation leading to B-cell apoptosis.
It follows that rituximab should play an important role in the management of AAV. Indeed, it is the only ‘kidney-related’ indication, for which the US – FDA has given approval (In April 2011).
Rituximab has been extensively tried in clinical trials for remission induction as well as maintenance. Some of the most prominent ones are discussed here
INDUCTION TRIALS
- RITUXVAS (2010)
- RAVE (2010)
MAINTENANCE TRIALS
- MAINRITSAN (2014)
- MAINRITSAN 2 (2018)
- RITAZAREM (2019) (Induction was also achieved with Rituximab)
- MAINRITSAN 3 (2020)
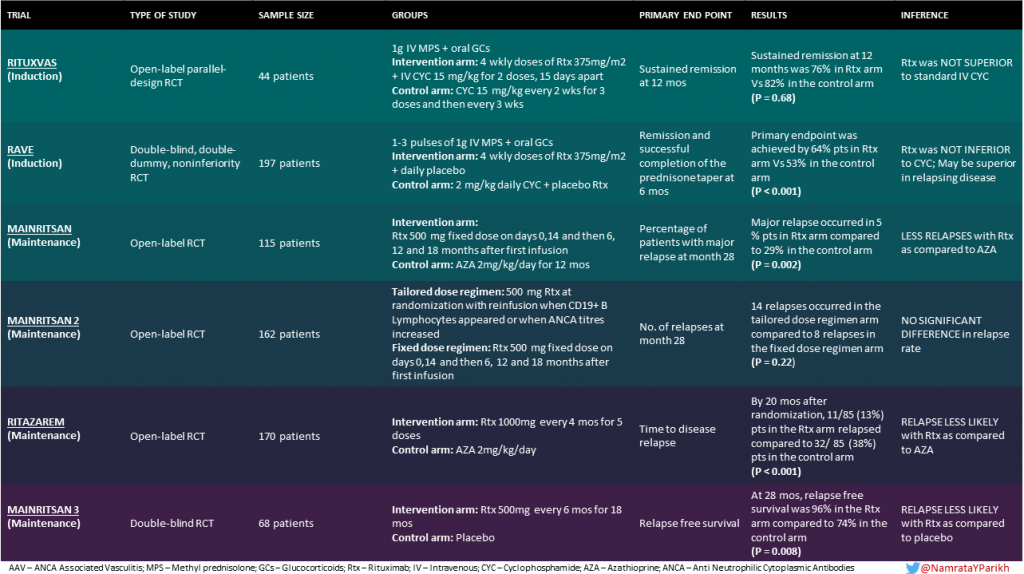
RITUXVAS
This open-label trial studied the use of rituximab as an induction agent in 44 patients with newly diagnosed AAV with kidney involvement.
This study did take the step of involving older individuals with a median age of 68 years.
Before enrollment, patients could receive plasma exchange or intravenous (IV) methylprednisolone, according to disease severity. After randomization, all patients were given 1 gram IV methylprednisolone and then oral glucocorticoids. Patients in the intervention arm received rituximab 375 mg/m2 body surface area (BSA) per week, for 4 consecutive weeks, and IV cyclophosphamide 15 mg/kg with the first and third rituximab infusions; these patients did not receive azathioprine for maintenance (although they did receive glucocorticoids). Patients with progressive disease within the first 6 months were permitted a third dose of IV cyclophosphamide. Patients in the control arm received IV cyclophosphamide 15 mg/kg every two weeks for the first three doses, then every three weeks thereafter until stable remission was achieved. They also received azathioprine.
Further treatment with rituximab or cyclophosphamide was permitted in cases of relapse.
The primary outcomes were sustained remission and rates of severe adverse events at 12 months.
Similar rates of sustained remission (p = 0.68) were obtained in both groups (76% in the rituximab group vs 82% in the cyclophosphamide group). Severe adverse events occurred in 14 patients in the former (42%) and 4 patients in the later group (36%). It was concluded that rituximab was NOT SUPERIOR to cyclophosphamide.
RAVE
This was the study that placed rituximab in the center of the discussion for nephrologists. RAVE was a randomized, double-blind, double-dummy trial, involving 197 patients with newly diagnosed or relapsing AAV. It is important to mention that RAVE was designed as a non-inferiority trial. The intervention group received rituximab 375 mg/m2 BSA/week for 4 weeks plus daily placebo−cyclophosphamide. The control group received placebo−rituximab infusions plus daily cyclophosphamide (2 mg/kg). For the maintenance phase the rituximab group received placebos and the cyclophosphamide group could continue on cyclophosphamide or switch to azathioprine. Both groups received 1-3 pulses of 1000mg methylprednisolone , followed by prednisone at a dose of 1 mg/kg/day.
The primary endpoint was remission and successful completion of the prednisone taper at 6 months.
This trial showed that rituximab was non inferior to cyclophosphamide for induction therapy – rituximab remission rates were 64% versus 53% with cycloy. Although not a primary endpoint, in a prespecified subgroup analysis, it was found that in patients with relapsing disease, rituximab was superior at 6 months and 12 months, but this effect did not extend out to 18 months. It is important to mention that this trial excluded patients with serum creatinine > 4 mg/dL and patients with alveolar hemorrhage who required mechanical ventilation. Interestingly, the rituximab group had similar rates of adverse events to the cyclophosphamide group. Overall, the remission rate of this study cohort was lower than seen in previous studies – the authors attributed this to the strict 6 month steroid taper.
MAINRITSAN
In this trial, rituximab was studied as a maintenance agent. It was a non blinded randomized controlled trial with 115 patients having newly diagnosed or relapsing AAV in complete remission. For induction, IV methylprednisolone (500-1000 mg/day for 1-3 days) or IV cyclophosphamide (0.6 g/m2 BSA on days 0,14 and 28 and then 0.7 g/m2 BSA every 3 weeks for 3-6 doses) were used. After achieving remission, and within 1 month of the last cyclophosphamide pulse, eligible patients were randomized to either rituximab or azathioprine.
Patients in the intervention group received rituximab (fixed 500 mg IV) on days 0 and 14 after randomization, and then at months 6, 12, and 18 after the first infusion. Patients in the control group received azathioprine (2 mg/kg/day) for 12 months, followed by dose reduction (1.5 mg/kg/day for 6 months and then 1 mg/kg/day for 4 months). Prednisone was further tapered and then kept at a low dose for at least 18 months after randomization.
The primary endpoint was the percentage of patients with major relapse (reappearance or worsening of disease with a BVAS >0 and involvement of at least one major organ, a life-threatening manifestation, or both) at month 28.
At month 28, patients in the rituximab group had less relapses (5%) as compared to patients in the azathioprine group (29%) (P=0.002) as compared to the azathioprine group. Their NNT was also quite astonishing: 4 patients.
MAINRITSAN 2
After rituximab was established as an option for maintenance treatment, MAINRITSAN 2 tried to clarify if the timing of rituximab doses was a factor in ensuring relapse-free maintenance. This was an open-label, multicenter, randomized controlled trial including 162 patients with newly diagnosed or relapsing AAV, in complete remission after induction therapy with glucocorticoids and cyclophosphamide, rituximab or methotrexate.
Both intervention and control arms used rituximab, the difference being in the dosage protocol. One group of patients received a 500 mg rituximab infusion at randomization, with reinfusion only when CD19+ B lymphocytes or ANCA reappeared or ANCA titre rose markedly. Study visits were scheduled at enrolment, then every 3 months until the endpoint, 28 months after randomisation. At each visit, BVAS was calculated and blood samples, for CD19+ B lymphocytes and ANCA titres, were drawn from every patient. Thus, in this arm, treatment was individually tailored for each patient.
Controls received a fixed 500 mg rituximab infusion on days 0 and 14 post randomization, then 6, 12 and 18 months after the first infusion.
The primary endpoint at month 28 was the number of relapses, defined as reappearance or worsening of symptoms or BVAS>0
At 28 months, there were 22 relapses of which 14 were in the tailored regimen group and 8 were in the fixed schedule group (P = 0.22). Thus, there was no significant difference in the relapse rates between the two groups. Note that individually tailored-arm patients received fewer rituximab infusions.
RITAZAREM
RITAZAREM was an open-label RCT designed with the aim of comparing rituximab with the treatment standard azathioprine for the maintenance of remission. 170 patients with relapsing disease in complete remission after induction with rituximab (4 doses of 375mg/m2 BSA/week) and glucocorticoids were studied (compared to MAINRITSAN where newly diagnosed patients were first treated with cyclophosphamide induction).Rituximab 1000 mg every 4 months for 5 doses was compared with azathioprine 2 mg/kg/day.
Patients were followed for a minimum of 36 months, with the primary outcome being time to disease relapse
By 20 months after randomization, 11/85 (13%) patients in the rituximab group relapsed, compared to 32/85 (38%) patients in the azathioprine group. Thus, participants in the rituximab group were significantly less likely to relapse(hazard ratio 0.30).
MAINRITSAN 3 evaluated the efficacy of an extended rituximab maintenance regimen attempting to address the question of what the optimal duration of maintenance therapy with rituximab is. This was a multicenter, double-blind RCT, which enrolled patients who had successfully completed the MAINRITSAN 2 trial without any major relapses and were in complete remission after the first phase of maintenance therapy (patients had to have Birmingham vasculitis activity score (BVAS); version 3 of 0 for 18 months).
The primary endpoint was relapse-free survival at month 28.
68 patients with GPA and 29 with MPA were randomized to receive standard maintenance therapy with rituximab 500 mg or placebo, every 6 months for an additional 18 months (4 doses). At 28 months (after MAINRITSAN 2), the primary end point of relapse free survival was achieved in 96% patients who received Rituximab compared to 74% in the placebo arm (P=0.008). 24% of patients in the intervention arm had serious adverse events (such as infections, cardiac or thromboembolic complications) as compared to 30% in the placebo arm. The authors concluded that extended therapy with biannual rituximab infusions over 18 months was associated with a lower incidence of AAV relapse compared with standard maintenance therapy.
In recent years, the focus has been on steroid minimization and steroid avoidance regimes in a bid to avoid steroid-associated adverse events. In an observational study, researchers at the University College London Centre for Nephrology, United Kingdom, described a steroid free maintenance regimen in which induction treatment consisted of two doses of rituximab, 3 months of low-dose cyclophosphamide and oral glucocorticoids for 1-2 weeks. Maintenance treatment consisted of azathioprine or, if intolerant, mycophenolate mofetil, methotrexate or rituximab. 49 patients were included, with at least 12 months of follow-up in 46, all of whom achieved remission. ADVOCATE is another recent trial, designed to study the role of C5a receptor inhibitor, avacopan, in AAV. Patients were randomized to receive oral avacopan or oral prednisone. All patients received either cyclophosphamide (followed by azathioprine) or rituximab. Remission at week 26 (the first primary endpoint) was observed in 120 of 166 patients (72.3%) receiving avacopan and in 115 of 164 patients (70.1%) receiving prednisone. Sustained remission at week 52 (the second primary endpoint) was observed in 109 of 166 patients (65.7%) receiving avacopan and in 90 of 164 patients (54.9%) receiving prednisone.
Rituximab has emerged as an effective candidate in the management of ANCA associated vasculitis, both as an induction and a maintenance agent, which may be useful in avoiding glucocorticoids.
Summary
Although RITUXVAS did not prove superiority of rituximab over cyclophosphamide, it did establish rituximab as a real option in AAV induction. RAVE further established the non-inferiority of rituximab as an induction agent in AAV. MAINRITSAN was an important trial that highlighted the efficacy of rituximab as a maintenance agent witha fixed dose and schedule, while MAINRITSAN 2 confirmed that it produced similar results in a regimen tailored as per CD19+ B Lymphocyte count and ANCA titres. These findings were further expanded by the results of MAINRITSAN3 which demonstrated that extended maintenance therapy with rituximab had a lower incidence of AAV relapses. RITAZAREM showed us that continued therapy with rituximab for maintenance after induction appears to be a better strategy for preventing relapses than azathioprine..
Conclusion
Kidney involvement portends poor outcomes in patients with AAV. Rituximab has been studied in several randomized clinical trials and shows good efficacy both as an induction and a maintenance agent and also carries the advantage of avoiding the cytotoxic side effects associated with cyclophosphamide such as infertility. However, cyclophosphamide should be preferentially used in patients with associated alveolar haemorrhage or rapidly progressive/crescentic glomerulonephritis according to KDIGO guidelines.
What do the current guidelines say?
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines, published in 2012 recommended cyclophosphamide and corticosteroids as the first line treatment of AAV (1A). However, it also accepted the use of rituximab, in cases where cyclophosphamide was contraindicated (1B) as well as in resistant (1C) and relapsing cases. Recently, KDIGO released a draft of guidelines on glomerular diseases for public review. These updated guidelines recommend that corticosteroids in combination with cyclophosphamide or rituximab may be used as initial treatment of new-onset AAV (1B).
The European League Against Rheumatism in conjunction with the European Renal Association—European Dialysis and Transplant Association (ERA-EDTA), EULAR/ ERA-EDTA, published guidelines which recommended use of either cyclophosphamide (1A) or rituximab (1B) for remission induction and maintenance. If the initial regimen included cyclophosphamide, use of rituximab is recommended in refractory cases (1B).
Reviewed By: Rhea Bhargava, Dearbhla Kelly, Matt Sparks.



Brilliant !!!! Really well depicted !!