The trial was enriched to try and increase the rate of AKI – it included patients with an eGFR between 15 and 45 (non-diabetic) increasing to 60 in diabetics. The overall mean eGFR was 50 so perhaps there were not quite enough patients with advanced disease but given that more than 5000 patients were included in the study, it is hard to really draw the conclusion that it was underpowered for subgroups. Patients with AKI were also understandably excluded and it is unclear what the risk is in this subgroup of patients.
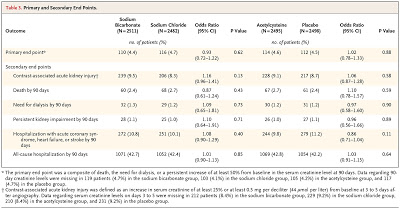
The other thing that was really interesting about this study was the outcome used. Traditionally, studies on CIN have used AKI as the outcome. This being defined typically as some change in creatinine in either absolute or percentage terms. The current AKIN definition of stage I AKI is a 0.3mg/dl increase. This study used an increase of 0.5mg/dl. AKI of this magnitude has been shown in large studies to be associated with adverse outcomes including increased length of hospitalization and mortality but there is always a lingering question about how clinically significant it is in the long run when it tends to resolve in most patients.
Because of these concerns, there has been a recent move towards using MAKE (major adverse kidney events) as a composite outcomes in trials of kidney disease. This concept, stolen somewhat from the cardiology literature is thought to be more meaningful as it results in real, long-term harm to patients. In this study, the authors chose MAKE90 – a composite of death, need for dialysis and permament 50% increase in creatinine at 90 days as the outcome. Overall, approximately 9% of patients had AKI following contrast administration and about 4.5% had a MAKE90 event (2.5% died, 1.5% required dialysis and 1% had a permanent decline in renal function). Another thing I would take away from this study is that we should not let people tell us that CIN does not exist (which I have heard around the halls more often than I like over the last year or so).
Overall, I think this move towards MAKE as an outcome in clinical trials is a welcome one. It is a well defined outcome that demonstrates clear harm to patients. There is no reason to chuck AKI out completely – it is a perfectly good secondary outcome – but in terms of figuring out who are the patients most likely to benefit from interventions in the future, MAKE is the way to go