APOL1 is the newest addition to the list of CKD risk factors- possibly never before in the history of nephrology has a gene associated with such a high odds ratio with disease – above 7 for hypertensive CKD & >10 for FSGS. A gene that confers heterozygous survival advantage when present as a single variant allele, but two variants lead to high CKD risk – APOL1 on chromosome 22. A similar picture of the survival advantage
APOL1 is the newest addition to the list of CKD risk factors- possibly never before in the history of nephrology has a gene associated with such a high odds ratio with disease – above 7 for hypertensive CKD & >10 for FSGS. A gene that confers heterozygous survival advantage when present as a single variant allele, but two variants lead to high CKD risk – APOL1 on chromosome 22. A similar picture of the survival advantageis seen with sickle cell disease. For more on the fascinating story of APOL1 itself, see this open access review, NephMadness coverage from 2015, and the NephJC coverage of a more recent research. A recent study has shown increased risk of CKD and subsequent ESKD in a population of black kidney donors with high risk APOL1 genotype. High risk is defined as presence of two variants (G1 and G2 – so any combination G1/G1, G1/G2 or G2/G2 is high risk) and was associated with faster progression of renal dysfunction and lower pre- and post-donation eGFR (pre-eGFR 98 versus 108; p= 0.03 and post-eGFR 58 versus 68; p=0.01) over a median follow up of 12 years. The second part of the study involved comparison between these donors and non donors from the CARDIA cohort (Coronary Artery Risk Development in Young Adults), based on APOL1 genotype status. After median 11 years, there was no difference in eGFR decline between the two groups when segregated based on genotype status, indicating that it was the genotype which influenced the eGFR decline and not the donation. Other salient features in the study were:
- 2 donors in the high risk group developed ESRD at 10 and 18 years of donation (11 %, but there were only 19 in this group).
- 78% of the donors were first degree relatives of the recipients. So the fact that a substantial percentage of donors were at risk of subsequent renal disease in this study does raises some valid concerns. Should all black donors be genotyped before transplantation?
An original article published in the Annals of Surgery recently studied eligible kidney donors (n=3438) from the CARDIA cohort of 1985-86 and deduced some risk scores based
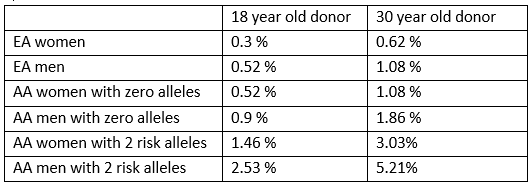
on the clinical and genetic profile of this population. They projected the 25 year pre-donation risk of CKD (eGFR <60ml/min) or microalbuminuria or macroalbuminuria in an 18 year old and a 30 year old potential donor. This risk is for people with no clinical risk factors (family history/pre-hypertension/diabetes). The risk increases significantly if any of these risk factors are present. See the table for some examples (EA– European American; AA – African American):
on the clinical and genetic profile of this population. They projected the 25 year pre-donation risk of CKD (eGFR <60ml/min) or microalbuminuria or macroalbuminuria in an 18 year old and a 30 year old potential donor. This risk is for people with no clinical risk factors (family history/pre-hypertension/diabetes). The risk increases significantly if any of these risk factors are present. See the table for some examples (EA– European American; AA – African American):
How about the risk to the recipients? Case reports have shown development of post transplant FSGS in siblings and monozygotic twins, both in the donor and in the recipient. Data from other studies have shown increased risk of allograft failure in recipients if the donor has a high risk genotype. All these data clearly point to the risk of CKD and subsequent ESKD in AA individuals (both donors & recipients) following transplantation. This brings us back to the most pertinent question, should people of African ancestry be genotyped for APOL1 prior to transplantation? The present studies are not well equipped to answer this question. Though it may seem that one obviously should screen (‘11% of donors with high risk variants develop ESRD!’ ‘High risk variants increase graft failure in recipients!’), lets pause and consider this. We know that live donor transplantation is the modality that offers the best survival. We also know that minorities, in US as well as in UK and elsewhere, have a lower rate of live donor transplantation (see NephJC coverage of a recent JAMA study and the ATTOM study, as well as the NephMadness coverage from 2017 on disparities in transplantation). So, would genotyping worsen the disparity? Is our fate inextricably written in our genes? Enter APOLLO.
APOLLO,
APOL1 Long-Term Kidney Transplantation Outcomes Network, is a prospective study aimed at genotyping donors and recipients in transplants involving recent African ancestry in United States and monitoring long term follow up. This will shed more light on this question and even might have a decisive influence on the present KDRI (kidney risk donor index), replacing the race column with APOL1 genotype. We await this important piece of work with interest.
APOL1 Long-Term Kidney Transplantation Outcomes Network, is a prospective study aimed at genotyping donors and recipients in transplants involving recent African ancestry in United States and monitoring long term follow up. This will shed more light on this question and even might have a decisive influence on the present KDRI (kidney risk donor index), replacing the race column with APOL1 genotype. We await this important piece of work with interest.
Post by Sriram Sriperumbuduri