In 2012, the introduction of the “monoclonal gammopathy of renal significance” (MGRS) for kidney disease associated with monoclonal proteins was revolutionary. MGRS was now clearly different from the benign monoclonal gammopathy of undetermined significance (MGUS). Prior to the identification of MGRS hematologists may have been hesitant to use cytotoxic therapy if patients did not meet traditional criteria for multiple myeloma or lymphoma.
A monoclonal protein or paraprotein (M-protein) is an immunoglobulin overproduced by an abnormally expanded clone of plasma cells. It can be a complete immunoglobulin, a light chain or more rarely, a heavy chain. The presence of an M-protein in the serum or urine indicates an underlying clonal plasma cell or lymphoproliferative disorder. In some cases, the clonal process producing the M-protein is malignant (multiple myeloma, Wäldenstrom macroglobulinemia). In other cases, the M-protein is produced by a small limited premalignant clonal expansion (MGUS or MGRS).
The landmark study done prior to the recognition of MGRS by the Mayo clinic reported a MGUS prevalence of 3.2% in people aged > 50 years, 5% in persons aged >70 years and as high as 8% in men aged >80 years. It has been reported that 37% of patients with an MGUS who underwent a kidney biopsy had a kidney disease associated with the M-protein. Although some plasma cell proliferative disorders present with very high M-protein concentrations, others, like MGRS, have little or virtually no circulating M-protein. It is this what makes this clonal marker so interesting and challenging.
More than ever, nephrologists must be aware of this “new” group of kidney diseases and perform appropriate investigations to be able to make these diagnoses. In order to achieve this objective we have to be familiar with the most common tests used for the identification of M-proteins (Table 1).
Table 1. Summary of 5 Tests to Find Monoclonal (M) Proteins. (Se: sensitivity; AL: AL amyloidosis; MM: multiple myeloma; LCDD: light chain deposition disease; MGUS: monoclonal gammopathy of unknown significance; MGRS: monoclonal gammopathy of renal significance)
- Serum protein electrophoresis (SPEP)
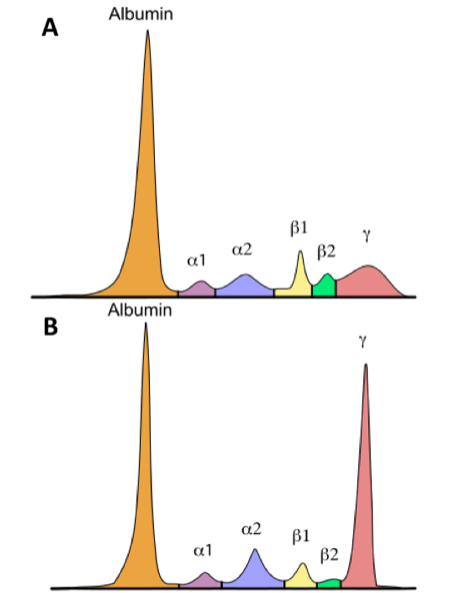
This test separates proteins by size in an agarose gel using an electrical current followed by quantification of the proteins using densitometer tracing of the gel. The proteins migrate into six zones: albumin, α1, α2, β1, β2, and γ. The β fraction often has two peaks in normal serum. Albumin is the most abundant protein in the serum and should make up the largest peak.
When M-protein is present, a tall peak or band appears in the γ region. M-proteins migrate together according to size and the peak may be found in the β fraction (up to 30% of IgA) or other fractions. When the M-protein is comprised of a small protein such as a free light chain (FLC) or the burden of the M-proteins is very low, the observable peak won’t be as tall or even not visible at all. Therefore an experienced clinician or chemist is needed for the correct interpretation of this test.
This is a inexpensive test and it is useful for diagnosis and follow up (if the amount of M-protein is large enough). The main caveat is that SPEP doesn’t identify monoclonality (for this immunofixation is needed), in other words it doesn’t detect the type of abnormal protein (complete antibody vs light or heavy chain). It can only detect and quantify spikes in the different zones, especially the β2 and γ. The limit of M-protein detection for SPEP is 0.3 – 0.5 g/dL in the γ region, therefore it won’t detect smaller amounts of M-proteins. It has a different sensitivity according to the underlying disease (Table 1).
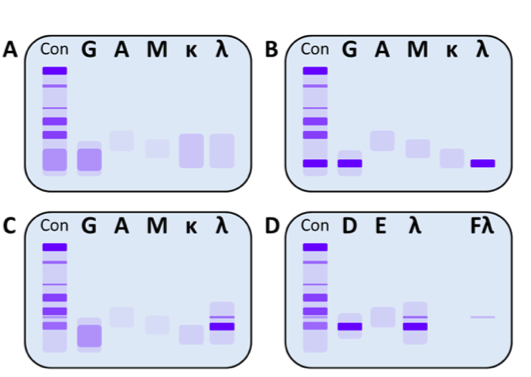
2. Immunofixation (IFE)
In this test a protein electrophoresis is performed in 5 parallel lanes: 3 for the heavy chains (G, A, M) and 2 for the light chains (κ, λ). Then antibodies specific for the different immunoglobulins are added to each lane. When positive, a dark band will appear on 2 lanes, one for the heavy chain and 1 for the corresponding light chain. Occasionally we can detect a monoclonal light chain without a corresponding heavy chain. In that case, the possibility of IgD and IgE M-protein must be excluded by using IgD and IgE antisera (Figure 2).
The detection limit for M-protein is 0.1 g/dL, therefore It can detect smaller monoclonal protein concentrations than SPEP. This is per se the most sensitive test with an sensitivity for the detection of M-proteins of 87%. Moreover, other advantage of IFE is its ability of discriminating the M-protein isotype, in other words, it can confirm monoclonality.
IFE is a qualitative test useful for diagnosis but not for follow up since the involved M-protein only needs to be identified once. Nevertheless, in order to determine complete response, IFE should be used because its greater sensitivity. In complete response, the M-protein should be undetectable by this method.
Ideally, SPEP and IFE should be done together at diagnosis as they complement each other. Combination of IFE and SPEP improves the sensitivity for detection of paraproteins (Table 1).
3. Serum Free Light Chains (FLC)
This quantitative assay uses antibodies directed against epitopes that are exposed only when the light chains are free (unbound to heavy chain) in serum. The normal FLC levels in the blood are kappa (κ): 3.3 – 19.4mg/L and lamda (λ): 5.7 to 26.3 mg/L. Therefore, a normal κ/λ ratio: 0.26 to 1.65.
This test is useful for the diagnosis of plasma cell dyscrasias that produce FLC, specially if the involved clone only produces FLC. It is most useful for detection of MM, AL amyloidosis and for monitoring treatment response (following the normalization of the κ/λ ratio). The major limitation of this test is that is not specific for monoclonal light chains. However, we infer monoclonality when an abnormal κ/λ ratio is found. The higher or lower the ratio, the more likely that a clone exists.
The introduction of this test in 2002 was a breakthrough because it allowed up to 67% of “non secretory” MM to be reclassified. This test alone has an overall sensitivity of 74% for detecting plasma cell dyscrasias and associated diseases. In combination with the previous tests, it improves the sensitivity for the diagnosis for MM to 100% and significantly improves detection of AL amyloidosis, MGRS, and LCDD (Table 1).
Of note, FLC are cleared by the kidney by filtration and reticuloendothelial system catabolism. The κ chains are produced at approximately twice the rate of λ chains but they are more readily eliminated by the kidneys. λ chains have a higher molecular weight and lower kidney clearance. In renal impairment (acute or chronic), the clearance of FLC is reduced. As the glomerular filtration rate decreases, the FLC ratio moves toward the higher κ production rate. Therefore, in severe renal impairment the κ/λ ratio must be adjusted to 0.37 to 3.1
Currently there are two major assays for the determination of FLC: the N Latex assay and FreeLite assay. Knowing which serum free light-chain assay is being used by the laboratory is important because the N Latex assay is the least affected by kidney function. Thus, the adjusting κ/λ ratio using this method may not be necessary.
4. Urine protein electrophoresis and urine immunofixation (UPEP and uIFE)
UPEP is analogous to SPEP and is used to quantify M-proteins (Bence Jones) in a 24h urine recollection. If a M-protein spike is identified, monoclonality should be confirmed by urinary IFE. It can detect a urine M-protein of 0.004 g/dL, nevertheless It has the lowest sensitivity of all; used alone it detects only in 37.7% of plasma cell dyscrasias. Since the introduction of the FLC assay, urinary studies have fallen out of favor. However, in combination with the serological tests, sensitivity for MGUS improves (up to 100%) and LCDD (from 77.8 to 83.3%).
On the other hand, the UPEP/uIFE provides us the total urinary protein level, urinary albumin proportion and M-protein isotype. The presence of an M-protein in urine with low urinary albumin suggests cast nephropathy. A high urinary albumin concentration suggest AL amyloidosis, LCDD or conditions that disrupt the glomerular base membrane. Hence, this test is an integral part of the evaluation of MGRS.
5. Urinary FLC Assay
Urinary FLC excretion does not always increase in patients with elevated serum FLC. For this reason, this test does not improve diagnostic sensitivity and its usefulness is unclear.
For more, check out @guptaarjun90’s tweetorial on this subject.
Post by: Alejandro Meraz
Onconephrology fellow at UHN, Toronto, Canada
#NSMC Intern, 2019
Thanks to Samira Farouk, Andrew Malone, Nikhil Shah, Matt Sparks for all the help.