Despite tremendous advancements in transplant and immunosuppressive therapy, transplantation in sensitized patients remains a major challenge. The presence of pre-transplant donor-specific antibodies (DSA) is strongly associated with poor outcomes, hyperacute, and antibody-mediated rejection. Sensitization can occur through blood transfusions, pregnancies, and prior transplant; and though it was once considered an immunological barrier to transplantation, it is now becoming a surmountable stumbling block. Many desensitization protocols have been developed to shorten the transplant waiting time of a sensitized recipient, especially for a deceased donor transplant. Current strategies rely on immunomodulation or antibody removal which may increase transplant rates and increase short term graft survival in sensitized patients. These protocols use preconditioning high‐dose intravenous immunoglobulins, or plasmapheresis combined with low‐dose immunoglobulins. Rituximab, eculizumab, tocilizumab, and bortezomib have also been used as they play a vital role in antibody depletion and cytokine inhibition. Still, the risk of antibody-mediated rejection remains high and long term graft survival is suboptimal.
There are no standard protocols for desensitization. They are usually determined by the anti-HLA characteristics and DSA levels. Patients are carefully monitored using crossmatch assays like complement‐dependent cytotoxicity, flow cytometry, and multiplex single antigen bead assays, before and after each cycle of treatment, to determine if the crossmatch has been rendered negative.
IdeS (Immunoglobulin G-degrading enzyme of Streptococcus pyogenes) or Imlifidase is an extracellular cysteine proteinase produced by S pyogenes.
It specifically cleaves all four human subclasses of IgG, generating one F(ab’)2 fragment and one Fc fragment, and thereby inhibiting both Fc-mediated phagocytosis and bacterial killing.
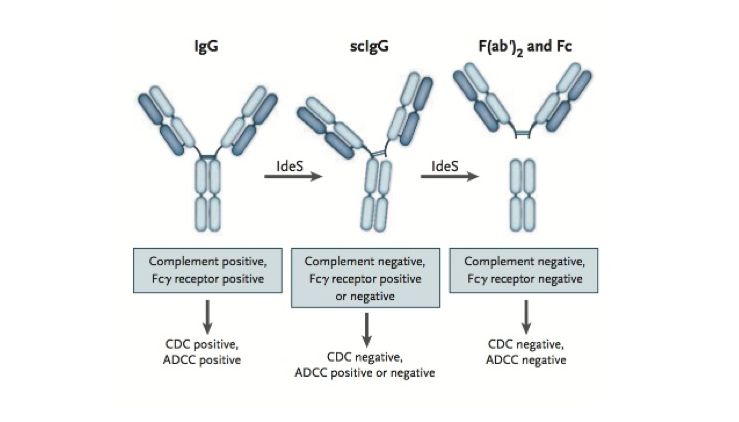
This IgG cleaving effect eliminates antibody-dependent and complement-mediated responses creating a therapeutic window for transplantation. IdeS can also cleave the IgG-type B cell receptor and compromise memory B cell activation and thus potentially counteract allograft rejection. Therefore IdeS may offer an alternative strategy to the already existing desensitization protocols.
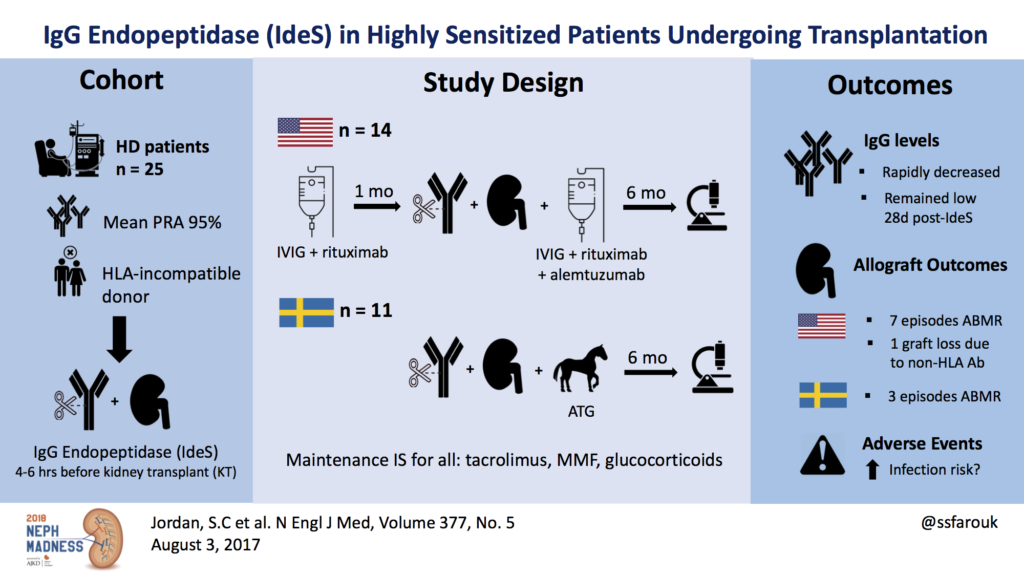
In a study published by Jordan et al (Fig. 3), the safety and efficacy of IdeS for the reduction or removal of DSA to allow HLA-incompatible kidney transplantation was assessed in an open-label phase 1 trial. All patients had evidence of sensitization, with a median cPRA (calculated panel reactive antibodies) concentration of 95%. One amongst the 25 patients had hyperacute rejection. DSA levels remained undetectable until two weeks post-transplantation when rebound occurred. Ten patients showed antibody-mediated rejection (ABMR) at two weeks to five months after transplant, but all were treated successfully. The acute rejection rate was 36%, which although high is comparable with other regimens using IVIg and rituximab. Lonze et al used IdeS in seven highly sensitized kidney transplant recipients (cPRA 98-100%) 24 hours prior to transplant. All patients became negative post IdeS, three developed antibodies rebound and ABMR which was successfully treated.
Currently, available data is insufficient to define the ideal population which would benefit from IdeS – whether it is DSA positive patients with only high risk of ABMR, or also those with low and intermediate risk. The optimal dosage of IdeS and the ideal combination of other immunosuppressive therapies to be co-administered remains unknown. IdeS may interfere with immunomodulatory drugs such as Anti-thymocyte globulin and tocilizumab causing antibody rebound and may also increase the risk of IgM triggered injury.
Apart from transplantation, IdeS is being tried in the treatment of anti-glomerular basement membrane disease, idiopathic thrombocytopenic purpura, Guillain-Barre syndrome, and certain malignancies.
Traditional desensitization protocols occur over days to months as compared with IdeS which may produce similar results with a single dose 24-hours prior to transplant. IdeS may, therefore, be an ideal drug in patients with more limited access to health-care.
As of October 2018, IdeS received fast track designation for transplant rejection prevention in the USA. The consistent ability of IdeS to eliminate anti-HLA antibodies makes it a promising addition to existing desensitization protocols and could help lower transplant waiting times. However, larger prospective trials with longer follow-ups are required to further support its use. A multicenter phase 2 trial of IgG endopeptidase for desensitization is currently ongoing (NCT02790437).
Post by: Krithika Mohan.
Nephrology fellow
#NSMC Intern 2019
Thanks to Maryam Khosravi, Divya Bajpai, Sarah Gleeson, Mayuri Trivedi and Matt Sparks for all the help.


