The last few decades have been revolutionary in terms of improving end stage kidney disease (ESKD) care with respect to increasing the number of transplant recipients as well as upping the immunosuppression game. The use of calcineurin inhibitors (CNIs) for maintenance immunosuppression has resulted in excellent short term graft outcomes, but long term outcomes have been associated with late graft loss and death. CNIs are known to increase cardiovascular morbidity and mortality and also have been implicated in causing chronic allograft nephropathy. There has been a concerted effort to find a long term immunosuppresive agent that successfully prevents rejection (and thus graft loss) and at the same time lacks adverse cardiovascular and kidney effects. Enter Belatacept.
The ability of helper T cells to recognize non-self antigens is critical for an effective immune response. In the context of antigen signal recognition,“Signal 1” is delivered through the T cell receptor after presentation of alloantigen bound to major histocompatibility complex (MHC) molecules on antigen presenting cells (APC). “Signal 2” or co-stimulation is initiated through the binding of CD80 and CD86 on APCs to CD28 and its homolog, CTLA4, on T cells. Belatacept, developed through fusion of CTLA4 with the Fc constant region of human immunoglobulin (Fcγ), blocks APC stimulation of T cell CD28, thereby inhibiting the immune response. Blockade of this stimulation inhibits T-cell activation, promoting anergy and apoptosis as compared to CNIs.
Various in vitro and in vivo studies have examined the efficacy of combined CD80/86 blockade. In a nonhuman primate model, Larsen et al’s landmark study demonstrated that belatacept monotherapy was inferior to combination therapy with belatacept and conventional immunosuppressive drugs in preventing allograft rejection. Importantly, belatacept prevented the development of donor-specific antibodies, which is believed to be a major contributor to chronic allograft loss in clinical settings.
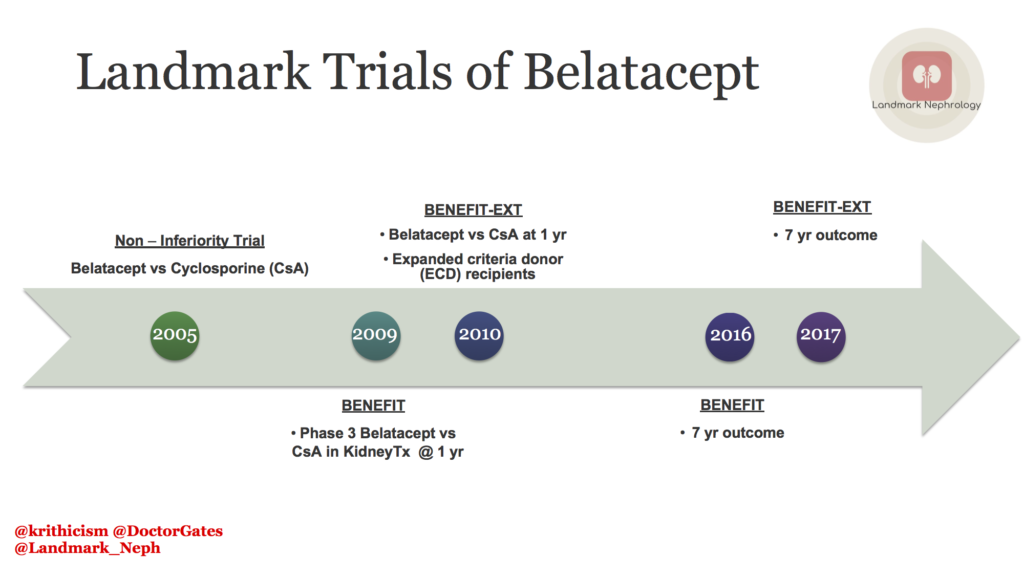
In 2005, Flavio Vinceti and colleagues published a non-inferiority study:which compared belatacept to cyclosporine (CsA). The primary outcome of the study was the incidence of acute rejection at 6 months after transplant. The rate of rejection in all the 3 arms was similar, although subclinical rejections were higher in the belatacept group.The efficacy of belatacept in kidney-transplant recipients was similar to that of CsA, but the potential of better preservation of eGFR, and a lower incidence of chronic allograft nephropathy. However, there was a higher incidence of post transplant lymphoproliferative disorder (PTLD) in epstein-barr virus (EBV) seronegative patients in the belatacept group.
The BENEFIT trial was a phase 3 RCT comparing the use of belatacept versus CsA for maintenance immunosuppression post kidney transplant. Patients who received belatacept experienced an unexpectedly higher incidence and grade of acute rejection episodes. The cardiovascular and metabolic profile (reduced blood pressure, lower incidence of diabetes and dyslipidemia) was better in the belatacept group as compared to the CsA group. However, PTLD was more common in the belatacept group. Despite a higher rate of early acute rejection, belatacept was associated with superior kidney function and similar patient/graft survival versus CsA at 1-year post transplant. BENEFIT was an open label trial, the primary endpoints were objective, and the causes of graft loss and death were adjudicated by independent external committees, therefore potential for bias cannot be excluded.
Long term effects of the BENEFIT trial were studied at 7 years after transplant. A significant reduction of 43% in the risk of death or graft loss was observed in the belatacept group as compared with the CsA group.The mean estimated glomerular filtration rate (eGFR) increased over the 7-year period with both belatacept regimens but declined with the CsA regimen. The cumulative frequency of serious adverse events at month 84 were similar across treatment groups. The authors, however, did not compare belatacept with tacrolimus, which is the current standard of care.
BENEFIT-EXT, was a phase 3 RCT, compared belatacept with CsA in higher risk extended criteria donor (ECD) kidney transplant recipients. ECD kidney transplant recipients treated with belatacept based immunosuppression achieved similar patient/graft survival, better kidney function, had an increased incidence of PTLD, and exhibited improvement in the cardiovascular/metabolic risk profile versus CsA treated patients. However, primary graft thrombosis as the adjudicated cause of graft loss occurred more frequently in the belatacept groups.
Long term effects of BENEFIT-EXT were evaluated at year 7 post transplant. Recipients of ECD kidneys randomized to belatacept had graft survival rates that were similar to those seen in patients randomized to CsA.The improvement in eGFR previously reported for belatacept versus CsA through 5 years of follow‐up was sustained and remained statistically significant at 7 years. The cumulative incidence of de novo donor specific antibodies (DSAs) was lower in each belatacept based treatment arm versus the CsA based comparator regimen. Rates of biopsy‐proven acute rejection were similar across treatment arms. Nevertheless, the risk of PTLD was greater among belatacept versus CsA treated patients, particularly in those who were EBV negative prior to transplant.
Although belatacept was associated with higher rejection rate, it did not translate into inferior long term outcomes. A potential benefit of belatacept ensuring compliance as it is a directly observed therapy by infusion. Currently, all studies have compared belatacept to CsA, and not the standard tacrolimus. Overall, the patient population in these trials were immunologically low risk and did not include sensitized recipients. The use of belatacept should be individualized, taking into account the immunological risk of the recipients while also balancing toxicities of immunosuppressive alternatives. Future studies will shed further light on the use of belatacept and its potential benefits on kidney transplant recipients.
Post by: Krithika Mohan, MD and Gates B Colbert, MD
Landmark Nephrology is an online learning tool designed to collect landmark trials in nephrology and distribute content that makes learning nephrology fun and easy.
Please visit us to check out our topic-specific content including videos, visual abstracts, quizzes, and a new slide-share portal to facilitate the exchange of educational material within the nephrology community.
We love collaboration so contact us as landmarknephrology@gmail.com or find us on Twitter to get involved!


