Drug related adverse events may not be uncovered until new therapies have been on the market for many years and used among a wider patient population than in the initial clinical trials. Downing and colleagues summarized that, during the period of 2001 to 2010, 32% of U.S. Food and Drug Administration (FDA) approved drugs had safety concerns within 6 years of initial approval. This indicates that both preclinical studies and multiphasic clinical trials likely fail to identify serious adverse effects. The last decade has seen the approval of targeted agents. A recent publication by Dr. Evren Azeloglu’s team from Mount Sinai pointed out that this may have implications for the development of chronic kidney disease (CKD), as acute nephrotoxicity, if untreated, could lead to gradual loss of kidney function. While some targeted anti-cancer drugs have been shown to have nephrotoxic effects, the mechanisms of therapeutic nephrotoxicity are diverse and not well-studied. In this blog post, we present a recent study by the Azeloglu lab, who uncovered a unique nephrotoxicity mechanism caused by dasatinib, a drug that has been on the market for more than a decade. Approved by the FDA in 2006, dasatinib is a multikinase inhibitor targeting the breakpoint cluster region-Abelson (Bcr-Abl) tyrosine kinase, vascular endothelial growth factor (VEGF), and Src kinases, which is used to treat chronic myelogenous leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL).
Recently, during a seminar session at our department, Dr. Azeloglu discussed his research on the nephrotoxicity of tyrosine kinase inhibitors, a group of drugs that were previously thought to be fairly safe to use. Dr. Azeloglu stated, “Since targeted protein kinase inhibitors are generally considered safe, most patients are not tested for kidney or glomerular function after the initiation of treatment.”However, based on his clinical practice, he was not fully convinced that the drugs were completely safe from a kidney standpoint, and outlined his methodological approach to the problem, starting with the FDA Adverse Events Reporting System (FAERS).
FAERS is the FDA’s post-marketing safety surveillance program for drug and therapeutic biologic products. It collects adverse event reports, medication error reports and product quality complaints resulting in adverse events. However, FAERS does not allow absolute quantification of adverse drug reaction (ADR) risk but only correlation of the ADR reported to the drugs. First, in an unbiased screen of the ADR on various therapeutic kinase inhibitors, they identified significantly higher reporting odds ratios (RORs) for nephrotoxicity. They continued to compute the RORs by factoring the frequency of ADR in subcategories to each of the kinase inhibitors of interest. The group found that dasatinib use was associated with increased risk of glomerulonephritis and proteinuria (from FAERS database) which was independent from the risk of hypertension. In addition, the RORs of dasatinib ranked higher in the categories of glomerulonephritis and nephrotic syndrome than tubular disorders and tubular necrosis on FAERS database which suggested the site-specific toxicity of dasatinib.
Now, they needed an in vitro model to hone in on the mechanism of such glomerular injury. Using immortalized mouse podocyte cell line derived from Mundel lab, they observed loss of actin stress fibers and focal adhesions with dasatinib exposure. Due to decreased cell viability and Src activity during dasatinib treatment, which was also observed during treatment with bosutinib (a potent Src inhibitor), they set out to test if dasatinib induces glomerular nephrotoxicity at a clinically relevant dosage through Src inhibition. By analyzing phosphoproteome of mouse podocyte cell line after dasatinib treatment, they determined that 16% of the down-regulated phosphoproteins were actin-binding proteins, and that the Src-regulated adhesion protein paxillin was at the top of this list.
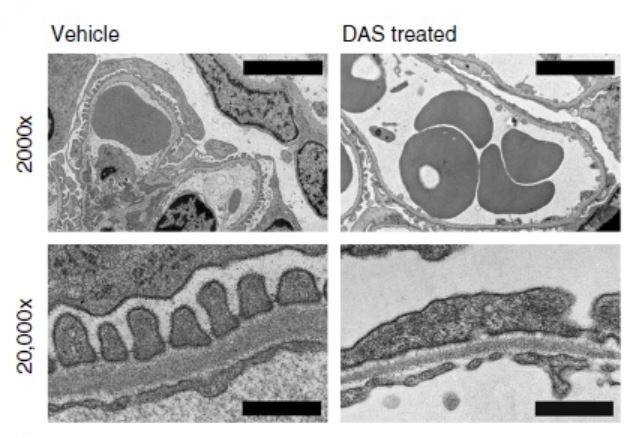
Next, they utilized automated microscopy and image analysis for quantification of changes in the actin cytoskeleton organization and focal adhesion morphology. They concluded that the impact of dasatinib on podocyte morphology/cytoskeleton was observed at a lower dose than its effect on viability, indicating that the cytoskeleton and focal adhesions of podocytes may represent key targets of dasatinib nephrotoxicity. They further quantified podocyte biomechanical changes by measuring cell stiffness with atomic force microscopy (AFM), in which a probe is used to “poke” the cell surface. The reduction in podocyte resistance to deformation under the condition of dasatinib treatment could lead to the loss of podocyte integrity. In normal mice treated with dasatinib, podocytes exhibited focal foot process effacement (flattening) by electron microscopy and significant synaptopodin expression reduction, although the number of podocytes was not reduced. The mice had moderate and irregular proteinuria after 5 weeks of dasatinib administration. This mild proteinuria was in agreement with the fact that the drug is generally well tolerated in clinical practice, although it is clear from the animal data that at least in some patients repeated administration of the drug can lead to more significant disruption of podocyte structure. MRL/MpJ Lupus mice had been shown to present actin cytoskeleton compromised podocytes. In order to replicate a more clinically relevant disease model, they switched to MRL/MpJ Lupus mice, which developed significant, although reversible, proteinuria after 4 weeks of dasatinib administration. They continued to hunt for potential targets for dasatinib-induced cytoskeletal instability by searching through the KINOMEscan dataset and identified the PAK-LIMK-cofilin pathway, which is important for actin cytoskeletal regulation, as a unique pathway impacted by dasatinib.
Among all the FDA approved targeted anti-cancer kinase inhibitors tested in this study, some VEGF receptor inhibitors (sorafenib, axitinib, sunitinib and pazopanib) presented the highest risk of nephropathies; however, they also had the highest risk of hypertension. Therefore, proteinuria caused by these drugs could be a secondary complication of vascular toxicity initiated by hypertension. Among the rest of the kinase inhibitors with a low risk of hypertension, imatinib, nilotinib, bosutinib, vandetanib, and erlotinib did not show stress fiber and focal adhesion abnormality in podocytes by automated microscopy and image analysis. This evidence suggests that actin cytoskeleton instability occurs after dasatinib therapy and could be responsible for the glomerular injury.
In conclusion, adverse effects of targeted therapies are increasingly being identified in a wide variety on novel therapeutics. This study provides an insight into discovery of pathophysiologic pathways behind adverse effects in the kidney. Such studies can help improve drug dosage protocols and potentially help screen for nephrotoxicity in susceptible patients.
The group is going to expand their effort to examine cytoskeletal signaling in patient samples and to develop patient-specific drug toxicity screening by using human iPSCs. The results of the above mentioned study was published in Nature Communications on May 3, 2019.
Dr. Azeloglu’s lab is currently hiring curiosity-driven postdocs! Candidates interested in integrative podocyte biology can contact Dr. Azeloglu for more detailed information.
Pei-Ju Liu
SUNY Upstate Medical University


