Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in the Caucasian adult population with an estimated incidence of 8–10 cases per 1 million and commonly seen in males above the age of 40. It is an immune-mediated disease that is pathologically characterized by thickening of the glomerular basement membrane and granular subepithelial deposits of immunoglobulins (Ig, usually IgG4 and complement (C3) along the capillary walls. It is typically characterized by subnephrotic to nephrotic-range proteinuria, edema, hypoalbuminemia, and hyperlipidaemia. Approximately 75%–80% of patients have primary membranous nephropathy (with the absence of any identifiable cause) and the remaining 20%–25% of patients have an identifiable secondary cause.
Spontaneous remission is a well-known characteristic of the disease but patients with persistent nephrotic range are at risk of progression to end stage renal disease and may require immunosuppression therapy. Over the years, many different immunosuppressive regimens have been studied.
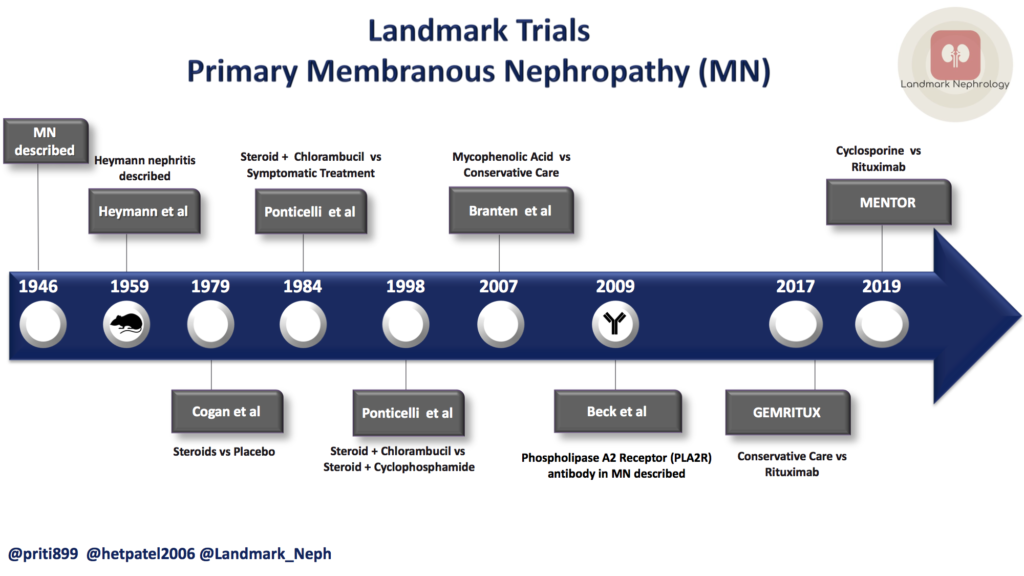
This post reviews the landmark studies that have shaped our modern-day management of this condition.
PLA2R Antibody in the Diagnosis and Management of Primary Membranous Nephropathy
Initial studies of membranous nephropathy used the Heymann nephritis animal model to demonstrate that the subepithelial immune deposits that are characteristic of the condition are formed in situ, but the target antigen was unknown. In 2009, Beck et al. identified the M-type phospholipase A2 receptor 1 (PLA2R1) as the target antigen in 70% of PMN cases. Interestingly, because these receptors constantly undergo endocytic recycling, they provide an ongoing source of surface-accessible antigen. The PLA2R antibody (as it is commonly referred to in the Nephrology community) can be detected in serum as well as in renal biopsy specimens and thus the discovery of this antibody has revolutionized the diagnosis and monitoring of primary membranous nephropathy. The presence of the PLA2R antibody makes the diagnosis of primary membranous nephropathy much more likely (though evaluation for secondary causes is still recommended), and serial monitoring of antibody titres has served as a marker to guide treatment. Clinically, decreasing antibody titres suggest immunological remission which is generally followed by remission of proteinuria. Beck and Salant also observed that titres of PLA2R antibodies may become undetectable prior to complete remission of proteinuria.
Treatment Options
Although spontaneous remission of primary membranous nephropathy occurs in approximately 30% of patients, patients with nephrotic syndrome who do not undergo remission are at risk of progression to end-stage renal disease. Kidney Disease Improving Global Outcomes (KDIGO) recommends initial treatment with an ACE inhibitor (ACEi) or angiotensin receptor II blocker (ARB) as well as optimal management of hypertension, hyperlipidaemia, edema, hypercoagulability, and infection risk. Patients with persistent nephrotic-range proteinuria may require immunosuppressive therapy and given the variable clinical course of the disease and often indolent progression rate, monitoring disease progression and balancing risks and benefits of treatment can be challenging.
1. Steroids
In 1979, Coggins et al, compared steroids with placebo. They demonstrated that in patients with nephrotic syndrome and preserved renal function, use of steroids for at least 8 weeks slowed deterioration of glomerular filtration rate (GFR). Steroids are now commonly used in treatment of PMN, in combination with other agents discussed below.
2. Alkylating therapy
In 1984, Ponticelli et al showed that preservation of renal function for at least 2 years was superior in patients treated with alkylating therapy (chlorambucil) alternated with steroids, compared to supportive care alone. Though the comparison was not to steroid therapy, the use of steroid therapy alone fell out of favour. By 1998, Ponticelli and colleagues went on to compare chlorambucil with cyclophosphamide, with both groups receiving initial IV steroids plus oral steroids every other month. Both treatments were effective, and per study authors, both were limited by adverse effects including herpes zoster (in 2 of the 36 patients who received chlorambucil) and cancer (occurring in one patient in each treatment group).
3. Calcineurin inhibitors
Given the greater familiarity Nephrologists have with calcineurin inhibitors, both cyclosporine and tacrolimus have been used as potential treatments. It was seen that the combination of cyclosporine with steroids was superior to steroids alone. Evidence also supports the use of tacrolimus in inducing remission but no long-term remission data is available and a higher rate of relapse was seen on discontinuation. These studies highlight the fact that CNIs can be used as an alternative to alkylating agents if poorly tolerated.
Mycophenolic acid can also be considered in patients who cannot tolerate other agents. It was found effective in decreasing proteinuria and improving renal function but did not appear as effective or better tolerated than cyclophosphamide. Cyclosporine and mycophenolate mofetil failed to show superiority over alkylating agents.
4. Rituximab
Rituximab has been the focus of investigation recently in PMN and two major randomized control trials have garnered a lot of attention. Published in 2017, GEMRITUX trial compared two 375 mg/m2 doses of rituximab to non-immunosuppressive anti-proteinuric treatment (NIAT), in patients who had persistent nephrotic syndrome after 6 months of NIAT. At 6 months, more patients in the rituximab group than in the control group reached the primary endpoint, but this difference was not significant. By 17 months, however, the rate of complete or partial remission in rituximab group was almost twice that of controls (64.9% versus 34.2%). A visual summary of this trial is attached below.
In the recently published MENTOR TRIAL by Fervenza et al, the anti-CD20 approach with Rituximab was found superior to cyclosporine in patients with persistent nephrotic range proteinuria at 3 months. After 24 months of follow-up, 39 of 65 patients (60%) in the rituximab group had complete or partial remission, as compared with 13 of 65 patients (20%) in the cyclosporine group. Progressive loss of eGFR was also slower with rituximab probably because of chronic nephrotoxic effects caused by cyclosporine. Because of its remarkable safety profile, some nephrologists are more inclined to use rituximab as first-line therapy.
5. ACTH
The drug H.P. Acthar® Gel (ACTH) is a natural form of ACTH and is obtained from processing porcine pituitary gland. The efficacy and utility of this drug is being studied. In a pilot study in 2014 Hladunewich et al, evaluated its use in dose of 40 or 80 IU twice weekly in a pilot study and it was found effective in improvement in proteinuria, serum albumin, and lipid profile. Notably, there are no randomized studies showing efficacy of ACTH for treatment of membranous nephropathy when compared to placebo or other interventions.
Check out some thoughts from #NephTwitter on ACTH in primary MN here.
Post by:
Priti Meena
Het Patel
Landmark Nephrology is an online learning tool designed to collect landmark trials in nephrology and distribute content that makes learning nephrology fun and easy.
Please visit us to check out our topic-specific content including videos, visual abstracts, quizzes, and a new slide-share portal to facilitate the exchange of educational material within the nephrology community.
We love collaboration so contact us as landmarknephrology@gmail.com or find us on Twitter to get involved!



So useful, thanks
Excellent article, very well explained.