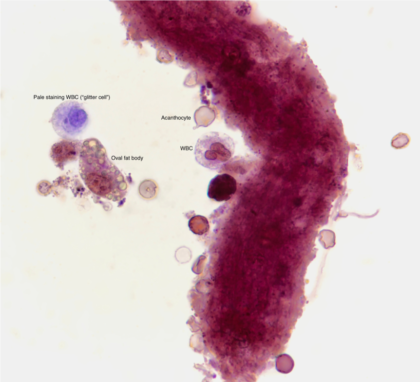
We continue a focus on features of myeloma kidney. Previous posts available here.
Monoclonal immunoglobulin deposition diseases (MIDD) are systemic disorders characterized by deposition of monoclonal immunoglobulins involving all components of the renal parenchyma including glomerular and tubular basement membrane, and mesangium. The deposits may be composed of either monoclonal light chains [Light chain deposition disease (~75%)-LCDD], heavy chains [Heavy chain deposition disease (~14%)-HCDD], or light and heavy chains [Light and heavy chain deposition disease (11%)-LHCDD].
Clinical presentation
The age of the patients ranges from 5th to 6th decade. Most of the patients (>90%) have proteinuria and nephrotic range proteinuria is present in ~40% of the cases. Underlying multiple myeloma is present in ~40% of the patients; the rest have either plasmacytosis or normal bone marrow biopsy. M spike is present in the serum or urine in majority of the patients, but ~15-20% of the patients have no detectable monoclonal proteins in the serum or urine. Hypocomplementemia may be seen in HCDD with complement-fixing IgG subclasses, IgG1 and IgG3.
Pathogenesis
LCDD: Approximately 80% of affected patients have an abnormal kappa light chain. Increased hydrophobic amino acids in the variable region of the light chains, usually in the CDR1 and CDR3 regions, reduce protein solubility and enhance tissue deposition in LCDD. Some light chains with posttranslational glycosylation which are larger than normal light chains have also been associated with LCDD.
HCDD: It is due to deposition of truncated heavy chains with deletion of CH1 constant domain, preventing assembly with the light chains. These structurally abnormal heavy chains have a predisposition for tissue deposition, and are usually not found in the urine.
Light microscopy
The most characteristic finding in LCDD is nodular glomerulopathy which mimics the nodular glomerulosclerosis seen in diabetic nephropathy. The nodules are PAS positive and nonargyrophylic. There may be a wide spectrum of other glomerular morphologies including normal appearing glomeruli, mesangial, membranoproliferative, and crescentic pattern. There may be variable thickening of the capillary walls due to subendothelial deposition of light chains. The tubular basement membranes may be thickened due to deposition of light chains on the outer aspect of tubular basement membranes. Concurrent light chain cast nephropathy is present in ~30% of the cases. There may be thickening of the vessel walls due to light chain deposits. There may be interstitial inflammation associated with interstitial deposits.
The broad spectrum of glomerular lesions seen in LCDD is usually not seen in HCDD and the predominant morphology seen in majority of the cases is nodular glomerulosclerosis.
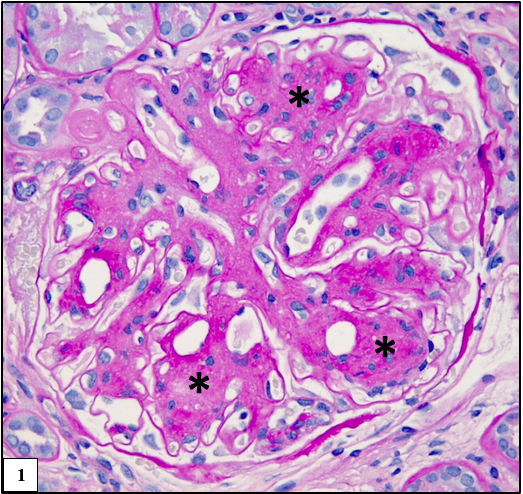
Figure 1: Glomerulus with nodular expansion of the mesangium (asterisk) which is similar to diabetic nodular glomerulosclerosis, Periodic acid-Schiff (PAS).
Immunofluorescence microscopy
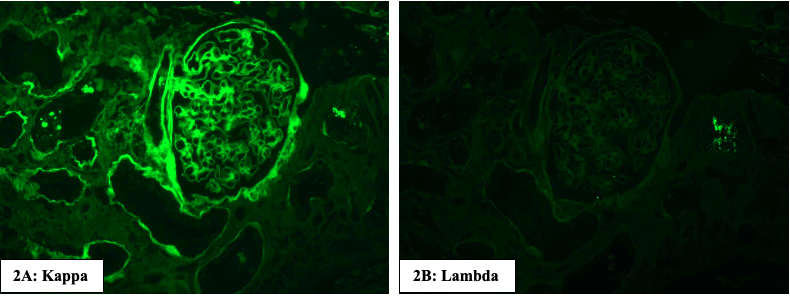
There is linear staining of the glomerular and tubular basement membranes, and the vessel walls for monoclonal immunoglobulins. The monoclonal light chain is most often of kappa isotype in LCDD and no staining is noted for the other light chain. There is absence of staining for immunoglobulin heavy chains and complements. In HCDD, there is staining for only one heavy chain and there is no staining for both the light chains. Usually γ heavy chain deposits are the most common and all the subclasses (1,2,3 & 4) have been reported. In LHCDD, there is deposition of one light and one heavy chain.
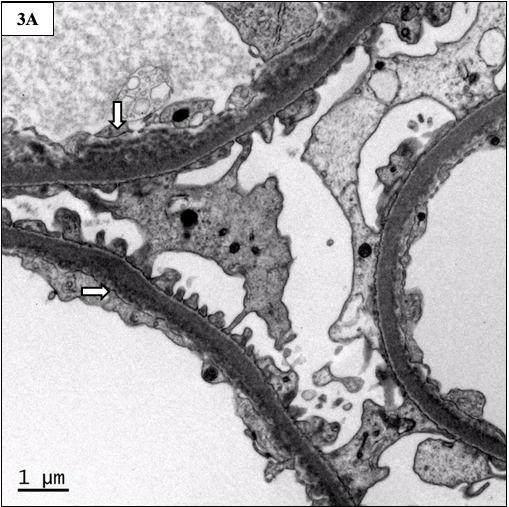
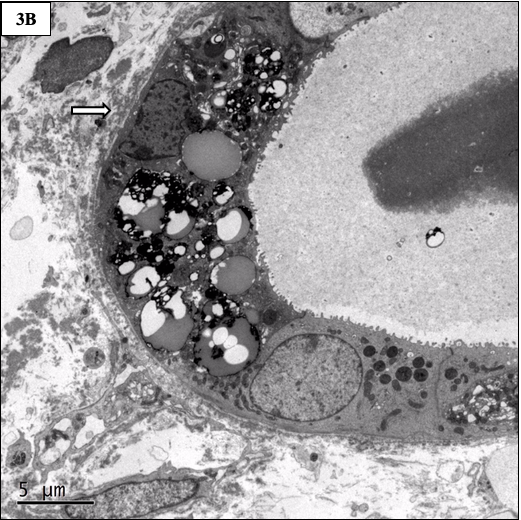
Electron microscopy
Finely granular to powdery deposits are present along the glomerular, tubular, and vascular basement membranes. The deposits appear similar in LCDD, HCDD, and LHCDD.
Differential diagnosis
Diseases with nodular pattern of glomerulosclerosis may also be seen in,
Amyloidosis: Congo red positive. Fibrils can be seen by electron microscopy. Concurrent amyloidosis is seen in ~13% of MIDD cases.
Diabetic nephropathy: Nodules are asymmetric compared to those of MIDD. There is absence of immunofluorescence staining or electron dense deposits by electron microscopy.
Idiopathic nodular glomerulosclerosis: usually related to smoking and hypertension. There is no history of diabetes. There is absence of immunofluorescence staining or electron dense deposits by electron microscopy.
Other entities in the differential diagnosis:
Proliferative Glomerulonephritis With Monoclonal IgG Deposits: Proliferative glomerulonephritispattern by light microscopy with monotypic IgG kappa or lambda staining by immunofluorescence. Electron microscopy shows amorphous electron-dense deposits in the glomeruli. There are no tubular basement membrane deposits by immunofluorescence and electron microscopy.
Fibrillary or Immunotactoid Glomerulonephritis: Diffuse mesangial expansion withfibrillary substructure by electron microscopy. There is absence of tubular basement membrane deposits.
Preethi Sekar, MD
Renal pathologist- Kovai Medical Center & Hospital, Coimbatore, India.[Picture courtesy-University of Chicago]