Although it remains a polarizing topic, looking at urine has come a long way since the dark ages before wide availability of renal biopsy. Not just a passing phase, modern urine microscopy allows the bright nephrologist to readily identify the elements of the urine sediment using various illumination techniques.
Bright-Field
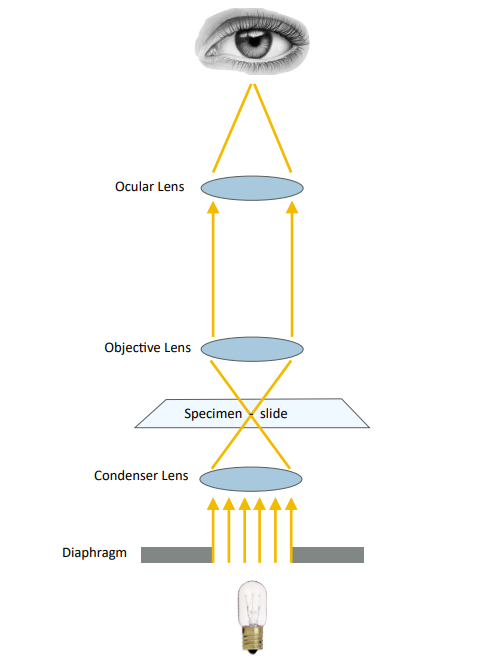
Bright-field illumination (Figure 1) is the simplest method, available on all microscopes. With this technique, all of the light from the light source passes through the condenser lens and is focused on the specimen, creating a dark image of the specimen against a bright background. For this modality the condenser turret should be set on “0” or “BF.”
Dark Field
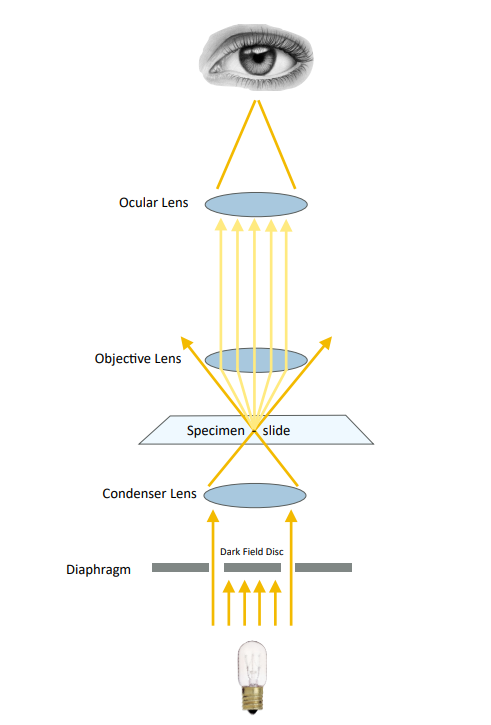
Dark-field illumination (Figure 2) is also available on most microscopes, as a result of an opaque disk in the condenser. In this modality, only the peripheral light from the light source is focused on the specimen creating a bright image against a dark background. Elements in the sediment with a high refractive index such as crystals and lipids appear especially “bright” under darkfield illumination. Set condenser to “DF.”
Phase contrast
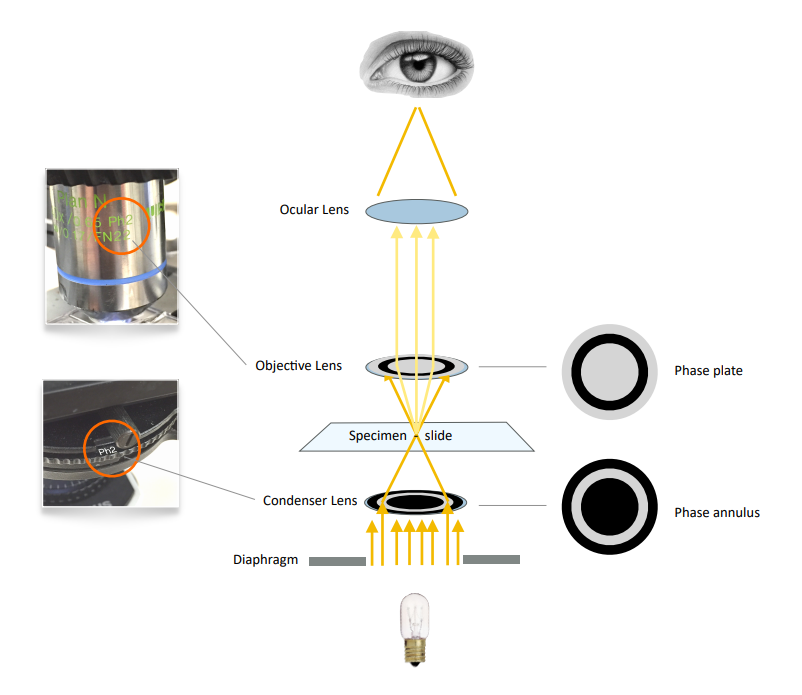
Phase contrast (Figure 3) alters the path of the light by using a “phase annulus” in the condenser and an inverse “phase plate” in the objective lens. The effect results in enhanced contrast of object edges and of elements with a low refractive index (such as hyaline protein matrix in casts). This modality requires a microscope fitted with a special condenser and
specific phase contrast objective lenses. Set condenser to “Ph1, Ph2, or Ph3” depending on which objective is in use.
Polarized Light
In polarized light microscopy (Figure 4) a polarizing filter (the polarizer) is placed below the condenser and another (the analyzer) in the optical pathway above the specimen (usually between the objective and the observation tubes). When the polarizer is turned 90° in relation to the analyzer all polarized light is blocked and the background of the image appears very dark. Light passing through anisotropic objects
on the slide (such as lipids, crystals, and various artifacts) is birefringent, meaning the light is refracted in two directions. The additional refracted light is able to pass through the analyzer resulting in a brightly contrasted object against the otherwise dark background. In other words, image contrast arises from the interaction of plane-polarized light with a
birefringent (or doubly-refracting) specimen to produce two individual wave components that are each polarized in mutually perpendicular planes.
Polarized light is useful for the identification of lipids, crystals, and contaminants such as starch and synthetic fibers. Certain lipids show a characteristic “maltese cross” patternmaking this modality very useful for identifying lipids in the urine.

polarization. Below, alternative method
placing analyzer on top of slide
It is often helpful to use all of these modalities. For instance (figure 5) , in the bright-field image on the top left we see what appear to be circular biconcave objects. Under dark-field in the top right image we note that these objects have a high refractive index, meaning they appear rather bright (typical of lipids and crystals). In the bottom left image under phase contrast we now note that these objects are actually within a cast. The
protein matrix of the cast was not visible under bright-field but is quite obvious under phase contrast. So now we are seeing circular inclusions within a cast but can’t tell yet whether these are RBC’s, lipid droplets, crystals, etc… But in the bottom right image, under polarized light, we see that these inclusions are birefringent and therefor crystals.
So what may not have been obvious under the initial brightfield image we now know are most likely calcium oxalate monohydrate crystals within a cast!
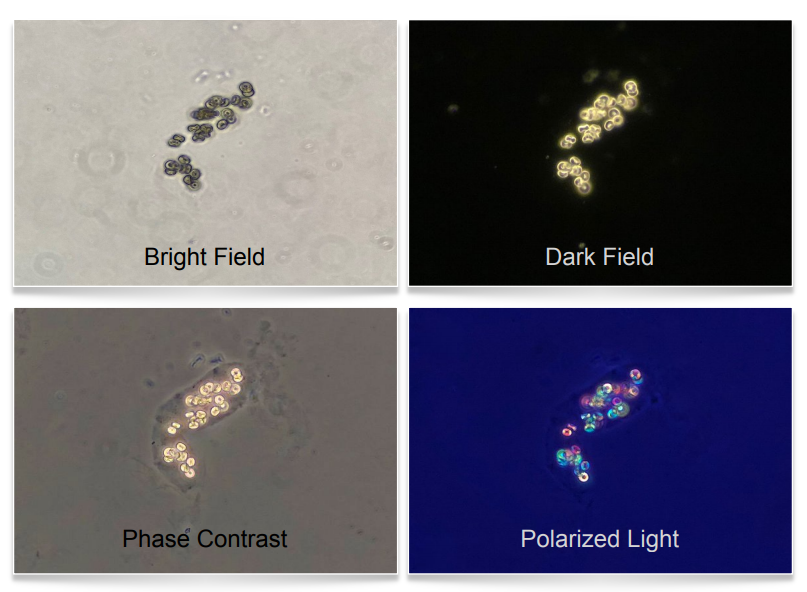
bright-field, dark-field, phase contrast,
and polarized light
In my experience, phase contrast offers the best balance of contrast and resolution with a thin prep of unstained sediment, however the highest resolution images are obtained with bright-field of a stained specimen. Phase contrast is not as good with stained specimens or crowded sediment. This is an interesting phenomenon, the result of enhanced contrast halos superimposed upon each other when the sediment is crowded, and stains causing unnatural contrast at the expense of resolution.
Dark-field is quite useful for low power scanning of a slide to readily identify casts, lipids, and crystals. It is also helpful identifying acanthocytes under higher power when phase contrast is not available.
Polarization allows you to verify the presence of lipids and assist in identification of crystals and artifact. As a part of your routine workflow remember to adjust the condenser focus and field diaphragm to achieve Köhler illumination (which focuses the light source exactly on the plane you are viewing end excludes extraneous light) and use the correct condenser turret setting for the illumination modality desired.
Post by Jay R Seltzer