Mythri Shankar, MD, DNB
Assistant Professor of Nephrology
Institute of Nephrourology, Bengaluru, India
@nephromythri
Membranous nephropathy (MN) is a common cause of nephrotic syndrome in n adults predominantly presenting in the 5th decade of life. The incidence of MN in adults is 12/million population/year. The incidence of kidney failure due to MN is 1.9/million/year.
The name “membranous” is derived from the fact that it is a disease of the glomerular basement membrane and “glomerulopathy or glomerulonephropathy” as there are no actively proliferating cells in the glomerulus and the pathology is mainly due to deposition of immune complexes. The “gold standard” for diagnosis is by kidney biopsy.
Primary Membranous Nephropathy: Under light microscopy (LM), we see thickening of the glomerular basement membrane with no proliferation or infiltration of inflammatory cells. Silver methenamine stains the glomerular basement membrane(GBM) and the “spikes”of GBM in between the immune deposits black. Under immunofluorescence (IF), granular IgG deposits along the glomerular basement membrane are seen. Electron microscopy (EM) shows electron dense subepithelial immune deposits and GBM projections in between the deposits.
MN is classified as primary membranous nephropathy (not associated with any underlying “other” disease) and secondary MN (associated with diseases such as malignancies, autoimmune diseases, infections, and drugs).
Pathogenesis:
There are three proposed mechanisms of MN:
- Antibodies against native podocyte antigens near or on the foot process
- Antibodies against “planted” antigens. These are cationic antigens present in the circulation that cross the anionic glomerular basement membrane barrier and get deposited in the subepithelial space. Usually explained as a cause in MN secondary to malignancies.
- Circulating antigen-antibody complexes deposit in the subepithelial space.
The search for the culprit antigen has been “on” since more than half a century and is ongoing. (Figure 1 )
Animal Model of MN:
Heymann nephritis model: first described by “Heymann” in the year 1959.
Megalin is an endocytic receptor on the podocyte foot process present in rats. It is absent in the human glomerulus. However, it is found in the brush border of PCT (proximal convoluted tubule) cells in humans.
- Active Heymann nephritis model: Immunizing rats with megalin triggered subepithelial deposits of immune complexes leading to nephrotic syndrome.
- Passive Heymann Nephritis model: Direct injection of anti-megalin antibodies, triggered subepithelial immune complex deposits.
These immune deposits activate the complements which injure the podocytes and hence, cause proteinuria.
Antigens in human MN:
Neutral endopeptidase (NEP)
For the first time, in the year 2002, an analogous mechanism in humans was studied in Paris. NEP is an antigen present on podocytes. It is involved in a rare, antenatal form of MN. Pregnant mothers who are genetically deficient in NEP, are sensitized to it during previous pregnancy, and develop antibodies against fetal NEP. These antibodies then cross the placenta and damage the fetal podocytes. The proteinuria in the fetus is transient and subsides a few months after birth.
Intracellular antigens
| IgG4 antibodies against the following antigens | % of patients with MN |
| Alpha enolase | 43% |
| Aldose reductase | 34% |
| Superoxide dismutase -2 | 28% |
Although they are intracellular, antibodies injure the podocytes causing translocation of these antigens to the cell surface where they are freely accessible to the circulating antibodies. Thus, amplifying the severity of injury.
Cationic bovine serum albumin (BSA)
As the intestinal tract in children is not completely developed, undigested or incompletely digested bovine milk proteins release antigens which are absorbed through the systemic circulation and get deposited in the glomerular capillary basement membrane as “planted antigens”.
Phospholipase A2 receptor (PLA2R)
M-type phospholipase A2 receptor is the predominant transmembrane antigen present in the glomerular podocytes.The M-type PLA2R was identified as a major antigen in human idiopathic MN in 2009.
Circulating Anti-PLA2R Ab:
- Anti-PLA2R antibodies are the major antibodies present in the circulation in the majority (70%) of primary MN. It is an IgG4 antibody which was identified by colocalization with anti PLA2R antibodies in kidney biopsy specimens.
Role of Anti-PLA2R Ab Titres:
- The level of anti PLA2R antibody titres have an important prognostic role. Low values suggested remission and higher values suggested prolonged course of proteinuria.Thus, emphasizing their role in monitoring disease activity.
- Most of the antibodies target a particular region on the antigen. Some studies demonstrated that the dominant epitope was within the N-terminal domain.
- Studies conducted later, proved that approximately 80% of the patients had antibodies binding to epitopes in first and seventh C-type lectin domains along with N-terminal ricin domain.This “epitope spreading” is associated with poor outcomes such as reduced rates of proteinuria remission, higher risk of progression to kidney failure and also reduced response to rituximab therapy.
Anti-PLA2R in kidney biopsy tissue staining:
- Immunofluorescence or immunohistochemistry staining for Anti-PLA2R antibodies in the renal biopsy specimen, is yet another way of identifying primary MN. However, circulating Anti PLA2R antibodies may be absent but tissue staining may be positive. Few mechanisms may explain this phenomenon:
- In the early stages, The kidneys “soak up” the circulating antibodies (the kidneys act as a “sink”). Hence, there aren’t sufficient amounts of antibodies in the circulation to be detected by the immunoassay.
- During spontaneous remission the circulating antibody titres reduce first followed by the clearing of immune deposits in the kidney. Hence, proteinuria lags behind circulating PLA2R antibodies.
Tissue PLA2R staining is more sensitive (69%-84%) than circulating PLA2R antibodies for the diagnosis of primary MN. Specificity is approximately 100%.
Anti-PLA2R antibodies have been reported in some of the secondary forms of MN as well, such as neoplasms, hepatitis B antigen positivity,sarcoidosis, use of NSAID. In such a scenario, it becomes difficult to differentiate primary from secondary forms of MN.Is primary MN coincidentally present is patients with these underlying diseases or is PLA2R also seen in some secondary forms of MN? We need more studies to answer this question.
Limitations:
The causal role of anti-PLA2R autoantibodies with disease has not yet been confirmed experimentally.
Thrombospondin Type 1 Domain containing 7A (THSD7A):
- Like PLA2R, THSD7A is a transmembrane protein present on the glomerular podocyte. It is a minor antigen responsible for primary MN in approximately 5% of the cases. It is predominantly seen in Japanese population. There are a few case reports of THSD7A positivity in MN secondary to malignancies.It was found that the circulating levels of THSD7A reduced after chemotherapy.
- There are a few case reports of MN with dual positivity (both THSD7A & anti PLA2R ). The clinical significance of this dual positivity is unknown. Its presentation is similar to other forms of primary MN.
The remaining 15% to 20% of the cases of primary MN are both PLA2R and THSD7A negative. So which are the other antigens hiding in there?
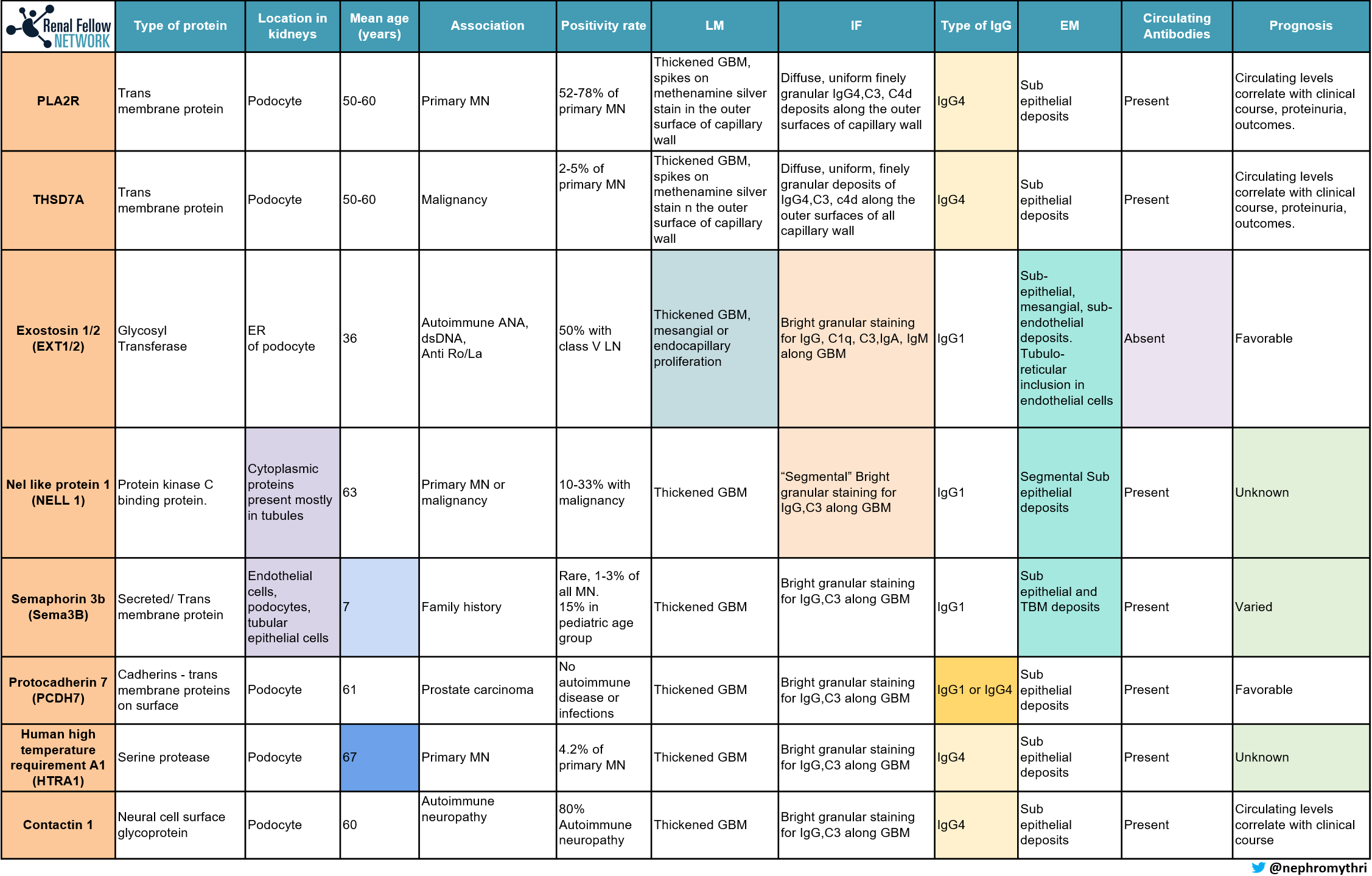
The use of laser microdissection and mass spectrometry (MS), has enabled the discovery of new antigens in MN. (see Table 1 below)
The order of incidence of these antigens:
PLA2R (Most common) > Exostosin > NELL1 > Protocadherin 7 > SEMA3B = THSD7A
Advantages of MS over IF (Immunofluorescence) / IHC (Immunohistochemistry)
- MS can identify all the possible antigens in one go, the spectral counts are higher of that particular antigen which is present and negative for the rest of the antigens.
- Identification of antigens by MS has higher sensitivity and specificity compared to IF/IHC.
- MS is less prone for technical errors.
- New antigens can be identified using MS
Limitations of MS:
- Longer turnaround time
- Expense and availability.
Hurdles in the way ahead:
- Although rare, how do we approach a case when more than one antigen is present?
- Can the use of MS replace IHC/ IF in the future?
- Antigens can be positive in both primary and secondary cases. So, how to go about treating such cases? As primary MN or secondary MN?
Edited by Matthew Sparks, Swasti Chaturvedi, Caitlyn Vlasschaert, Gerren Hobby, Sudha Mannemuddhu, Anju Yadav, Sophia Ambruso, Amy Yau, Anna Gaddy, Nayan Arora.



Hi Harsha,
you are detecting an antibody (ies) that are specific to PLA2R and NOT the endogenous PLA2R protein. It is true that protein could and is sometimes increased, but the disease occurs secondary to the antibodies being produced that are directed towards PLA2R and not the actual protein itself.
Very nice review. Summarises it very well. Easy to read.
I have a clarification with the following line though –
‘Immunofluorescence or immunohistochemistry staining for Anti-PLA2R antibodies in the renal biopsy specimen, is yet another way of identifying primary MN. However, circulating Anti PLA2R antibodies may be absent but tissue staining may be positive’
This makes it appear that what we are identifying on the biopsy is an antibody and not the antigen. My understanding is that PLA2r antigen is over expressed on the podocytes and detected with the help of an antibody.
Nice summary, suitable for educational purposes (….and beyond)
Good summary. Nice job, Mythri.
Sanjeev
Thanks for putting this together! It is a nice summary of the work to date … we will see what the future brings in terms of more antigens!
Verty good