Welcome to the 8th case of the Skeleton Key Group, a team of twenty odd nephrology fellows who work together to build a monthly education package for Renal Fellow Network. The cases are actual cases (without patient identifying information) that intrigued the treating fellow.
Written by: Jefferson L. Triozzi
Visual abstract by: Dominique Tomacruz
A. The Stem
A 45-year-old man with no known medical history and no prior medical care presented to the emergency room after a new-onset, self-limited generalized tonic-clonic seizure. At the time of evaluation, he was obtunded but not actively seizing. His vital signs and physical examination were largely unremarkable with no focal neurologic defects. He was unable to provide further history, and there were no friends, family, or witnesses available for interview.
B. The Labs
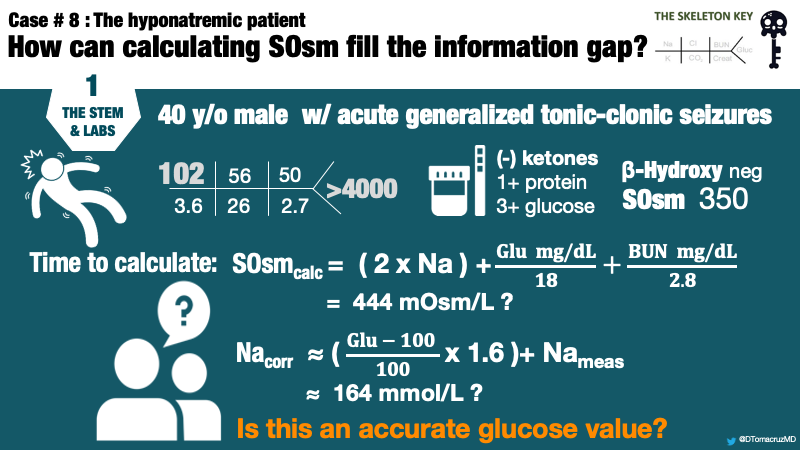
There were no previous labs for comparison. Based on the above lab values, the patient was determined to have a mixed anion gap metabolic acidosis and respiratory acidosis. There was no ketoacidosis, so the metabolic acidosis was attributed to lactic acidosis. The respiratory acidosis was attributed to obtunded mental status. Nephrology is consulted for hyponatremia.
C. Differential Diagnosis: Hyponatremia
The diagnosis of hyponatremia begins with an assessment of serum osmolality and tonicity.
Below are some key terms to get us started:
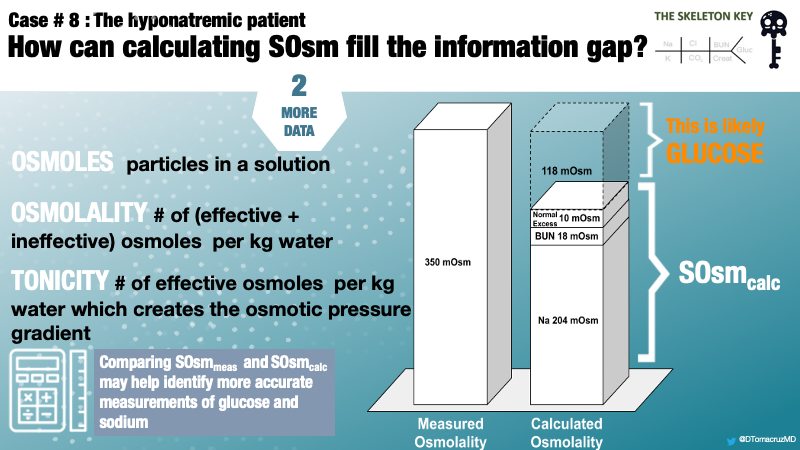
- Osmoles – the particles in a solution
- Osmolality – the number of all osmoles (both ineffective and effective) per kg of solution
- Osmolarity – the number of all osmoles (both ineffective and effective) liter of solution
- Osmosis – the movement of water from solutions of low solute concentration to high solute concentration
- Tonicity – the number of effective osmoles per kg of solution; it is tonicity which causes the osmotic movement of water, not osmolality
In most clinical scenarios, the difference in osmolarity and osmolality is insignificant.
Osmolality and Tonicity — Effective vs Ineffective Osmoles
Only osmoles which do not readily cross the semipermeable cellular membrane create an osmotic pressure gradient leading to water movement. These are “effective” osmoles and affect serum tonicity. For example, sodium is “effective” because it cannot readily cross the cell membrane, thereby creating an osmotic pressure gradient. On the other hand, urea, ethanol, glycine, and other alcohols easily cross the cell membrane and thereby are “ineffective” in creating an osmotic pressure gradient.
In the presence of adequate insulin, glucose is an “ineffective” osmole that is quickly consumed by cells through glucose transporters. In insulin deficiency, glucose acts as an “effective” osmole. By definition, any time a patient is hyperglycemic there is at minimum relative insulin deficiency.
Hyperosmolar Hyponatremia
Sodium is the primary osmotically active solute in serum and, thus, hyponatremia is usually a state of hyposmolality and hypotonicity. The initial evaluation of hyponatremic patients must assess serum osmolality.
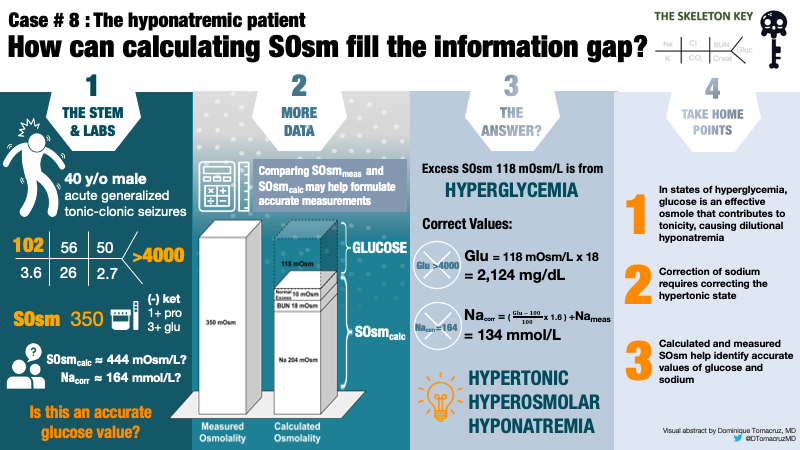
In this case, serum osmolality is 350 mOsm/kg, well above the normal range of 275 – 295 mOsm/kg. What we really care about is tonicity, because this affects cell volume. Whereas hypoosmolar hyponatremia is always a hypotonic state, non-hypoosmolar hyponatremia can be a hypo-, iso-, or hyper-tonic state.
A three-step approach can aid in evaluation of tonicity of non-hypoosmolar hyponatremia. First, assess for the presence of hyperglycemia, a common “effective” osmole. Second, assess for the presence of urea, ethanol, or other toxic alcohols, common “ineffective osmoles.” Third, if neither are present, consider pseudohyponatremia, a laboratory artifact due to an accumulation of cholesterol or proteins.
In this case, elevated blood glucose is the obvious excess osmole, contributing to both hyperosmolality and hypertonicity. There is no excess urea, ethanol, or or toxic ingestion by history or laboratory findings. Our suspicion for pseudohyponatremia is low with no obvious source of excess cholesterol or proteins.
D. More Data
Thus far, we have a case of hyperosmolar hyponatremia due to hyperglycemia in the face of an undetectably high serum glucose. Comparing measured and calculated osmolality will help formulate a more accurate measurement of serum glucose and, subsequently, corrected serum sodium.
Serum osmolality is measured in the laboratory based on the physical properties of a solution. Serum osmolality is calculated using an equation based on the measurements of the primary osmoles in serum: sodium (Na), glucose (Gluc), and blood urea nitrogen (BUN).
In this case, we are able to account for 204 mOsm/kg from sodium, 18 mOsm/kg from BUN, in addition to 10 mOsm/kg added for an expected normal osmol gap.
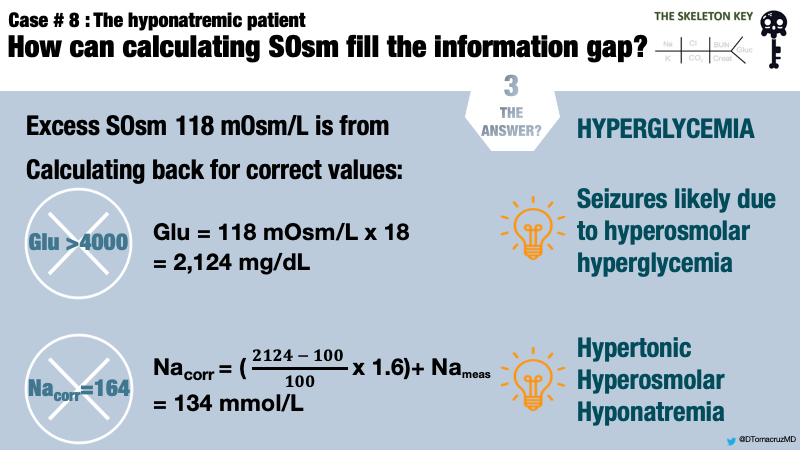
We have now calculated 232 mOsm/kg of a measured 350 mOsm/kg leaving a difference of 118 mOsm/kg.
In this case, there is no obvious reason for this osmolar difference other than serum glucose. Therefore, the remaining 118 mOsm/L can be attributed to blood glucose (Figure 1).
Using a correction factor of 18, the remaining 118 mOsm/kg yields a serum glucose estimation of 2,124 mg/dL. The Guiness World Record for highest blood glucose is 2,656 mg/dL. Although this is no Guiness World Record, it certainly is an extreme value of glucose. The laboratory reported value of >4000 mg/dL is a laboratory error.
But what is the tonicity? Tonicity is calculated by evaluating only the “effective” osmoles, sodium and glucose.
So our patient has a hypertonic hyperosmolar hyponatremia. We can now calculate the corrected Na for glucose, which is the expected Na concentration once hyperglycemia resolves, assuming a “closed system.” Traditionally, a factor of 1.6 mEq/L of additional sodium is added for every increase of serum glucose of 100mg/dL above a normal concentration of 100mg/dL.
Using the traditional factor, the corrected sodium will be 134 mEq/L. If the initial laboratory reading of glucose >4000 was taken at face value, serum sodium would be incorrectly estimated as >164 mEq/L.
E. The Answer: Hyperglycemia-Related Hyponatremia
This patient presented with hyperglycemic hyperosmolar state (HHS) in the setting of previously undiagnosed diabetes mellitus. The measured and calculated laboratory values are summarized below (Table 1).
F. How do we manage this?
Hyperglycemia-related hyponatremia results from the osmotic activity of glucose in the serum. Water shifts from the intracellular to the extracellular compartment, diluting sodium in the process. Following treatment with insulin, cells consume glucose and water shifts back to the intracellular compartment, correcting sodium in the process.
Hyperosmolar hyperglycemic state (HHS) is diagnosed in patients with hyperglycemia, altered mental status, and serum osmolality >320 mOsm/kg in the absence of ketoacidosis. Severe cases lead to organ failure, seizures, and death.The etiology of seizures and decreased level of consciousness is not entirely understood in HHS. Although there is a theoretical risk of cerebral edema as osmolality drops, cerebral edema is very rare in adults with hyperglycemic crises. Nevertheless, the American Diabetic Association (ADA) recommends lowering serum glucose by a steady rate of 50-70 mg/dl/hr and adding D5W to maintain blood glucose at 250-300 mg/dL until osmolality normalizes (Figure 2). These patients should be monitored closely with serial evaluations of corrected sodium, input and output, and frequent neurologic checks.
This patient was admitted to the medical intensive care unit and treated with intravenous insulin and isotonic crystalloid infusions. With normalization of hyperglycemia, his sodium increased to 134 mEq/L–exactly our estimation! His kidney function recovered. Hemoglobin A1c was 12.7%. Anti-pancreatic islet cell antibody and GAD-65 autoantibody levels were negative. His mental status improved and there were no further seizures. He was discharged on insulin therapy with a new diagnosis of type 2 diabetes mellitus.
Take Home Messages
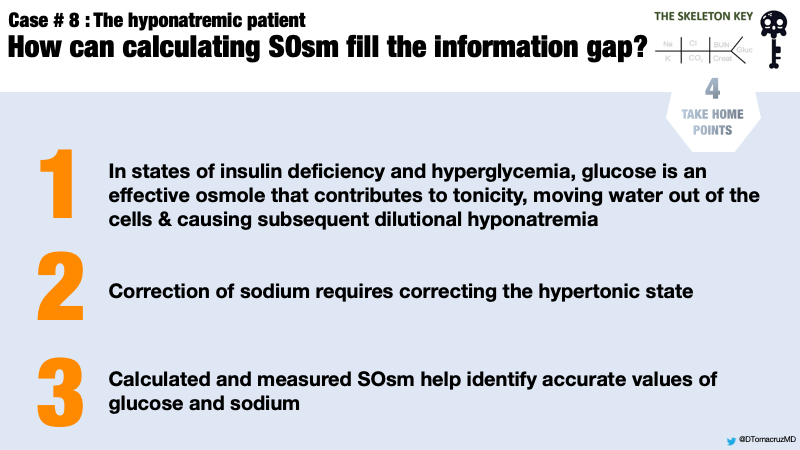
- In states of insulin deficiency/hyperglycemia, blood glucose is an effective osmole that contributes to both osmolality and tonicity.
- Hyperglycemia causes hyponatremia by shifting water from the intracellular to the extracellular compartment.
- Severe hyperglycemia-related hyponatremia is a medical emergency that depends on accurate laboratory measurements, including serum osmolality, to predict the corrected sodium.



Excellent case. I have a question: in this case does rate of sodium correction matter? In other words; this patient will get insulin and fluids and with correction of glucose sodium gets corrected too, but are we concerned for ODS? Do we need to still maintain sodium change 6-8meq? Or can we assume the corrected sodium to glucose is 134 anyway and patient is low risk for ODS and we don’t need to worry about the rate of correction?
Excellent case!! One of the most common clinical scenarios seen my hospital , explained in a beautiful way 🔆
Excellent teaching case!
Superb case
Good review. Thank you.
I have a question.
How long did it take to get this patient’s blood sugar back?
Theoretically, the calculated glucose was 2124, so was it true that it took about 26 hours if treated with 50-70 mg/dl/hr and adding D5W to maintain blood glucose at 250-300 mg/dL until osmolality normalizes?
Thank you. Every doctor must know this
Beautiful case
Nicely explained!!