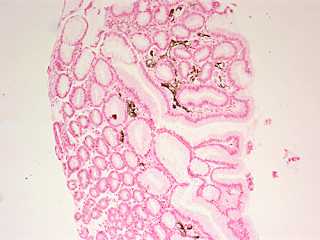
 One of the hot topics in Nephrology over the past few years has been the epidemiologic and histopathologic studies suggesting oral sodium phosphate colonoscopy preparations as a cause of acute phosphate nephropathy. As evidence continued to mount, the FDA in December 2008 forbade the over-the-counter sale of oral sodium phosphate products; however, they are still available by prescription under the names “Visicol” and “Osmoprep.” These preps are still favored by many gastroenterologists (and patients) based on the fact that it is much easier to take than the more traditional polyethylene glycol-based colonoscopy prep (e.g., “Go-Lytely”).
One of the hot topics in Nephrology over the past few years has been the epidemiologic and histopathologic studies suggesting oral sodium phosphate colonoscopy preparations as a cause of acute phosphate nephropathy. As evidence continued to mount, the FDA in December 2008 forbade the over-the-counter sale of oral sodium phosphate products; however, they are still available by prescription under the names “Visicol” and “Osmoprep.” These preps are still favored by many gastroenterologists (and patients) based on the fact that it is much easier to take than the more traditional polyethylene glycol-based colonoscopy prep (e.g., “Go-Lytely”).
Biopsies of patients with acute phosphate nephropathy tend to show abundant calcium phosphate crystal deposition, mostly within the distal convoluted tubules and collecting ducts, but sometimes also in the interstitium. A good detection method is the use of the von Kossa stain, which stains certain calcium-containing salts such as calcium phosphate a brownish-blackish color.

