 File “Gordon Syndrome” under “interesting causes of hyperkalemia and metabolic acidosis you may never see.”
File “Gordon Syndrome” under “interesting causes of hyperkalemia and metabolic acidosis you may never see.”
Also called pseudohypoaldosteronism type II, Gordon Syndrome is relevant less so for the number of patients afflicted but more due to the interesting insights into normal acid-base and electrolyte physiology.
Briefly, patients with Gordon Syndrome, a genetically-inherited condition, exhibit salt-sensitive hypertension, hyperkalemia, and a non-anion gap metabolic acidosis in association with a normal GFR. These metabolic derangements tend to be highly responsive to thiazide diuretics, correctly implying the disease is due to a constitutive activation of thiazide-sensitive Na channels in the distal convoluted tubule. In fact, Gordon Syndrome can be thought of as a mirror image of Gitelman’s Syndrome, in which there is inactivation of the thiazide-sensitive Na channels causing the exact opposite metabolic abnormalities (hypokalemia and metabolic alkalosis).
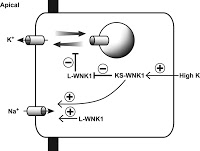
It turns out that Gordon Syndrome is caused by mutations in two different, related genes which encode for a type of kinase: either gain-of-function mutations in WNK1, or loss-of-function mutations in WNK4 (“WNK kinase” stands for “with no lysine kinase”). WNK4 is responsible for tonic inhibition of the thiazide-sensitive Na; its loss-of-function therefore results in unregulated Na reabsorption in the distal tubule. This leads to decreased Na delivery to the collecting duct, resulting in reduced tubular lumen electronegativity, the driving force for aldosterone-mediated potassium and H+ secretion. WNK1 is a negative regulator of WNK4 and this explains why gain-of-function in WNK1 can cause the same phenotype as loss-of-function in WNK4. Part of the clinical phenotype seen in these patients may also have to do with WNK effects on the potassium channel ROMK, illustrating the complex molecular biology of this pathway.

