Introduction
Dialysis Access-Associated Steal Syndrome (DASS) has been reported in up to 6% patients with an arteriovenous (AV) access. However, the true incidence of clinically significant DASS, requiring surgical intervention, may be lower as reported in a prospective cohort of over 600 hemodialysis patients. DASS is more commonly seen with brachial artery-based AV access compared to the radial artery-based AV access.
The main risk factors for developing DASS are as follows:
–AV access created from the brachial artery
–Diabetes Mellitus
–Female gender
–Coronary artery disease
–Peripheral vascular disease
–History of DASS with a prior AV access
Pathophysiology
To understand the etiology of DASS one should be familiar with the hemodynamic changes that occur after creation of an AV access. The estimated blood flow through the brachial artery is about 80 ml/min. A functional AV access generally has a blood flow of ~600 to 800 ml/min implying that there is almost a tenfold increase in blood flow after the AV access creation. To accommodate the high blood flow, there is dilation of the inflow artery and outflow vein mediated by release of nitric oxide.
From this point onwards, the hemodynamics are rather complex, and involve four vascular beds:
1. Artery feeding the AV access: Arterial Inflow
2. AV access: Outflow Resistance
3. Arterial supply distal to the arterial anastomosis of the AV access
4. Collateral arteries
For example:
1. If Inflow pressure is < distal arterial pressure (P < Q), there will be retrograde flow from the distal artery into the AV access,especially during diastole, leading to distal tissue ischemia.
2. If inflow pressure is adequate but peripheral arterial resistance is high (Q >> P), such as seen in atheroslcerotic vascular disease, there will be retrograde flow into the AV access during diastole
3. If the venous outflow resistance is low (R < Q), as seen in high flow AV access, there will be retrograde flow from the distal artery into the AV access leading to the phenomenon of ‘steal’.
Indeed, the hemodynamics involved are complex however, pressure imbalances invariably lead to retrograde flow from the distal artery towards the AV access in cases of DASS.
| Pathology | Causes |
| Inadequate Inflow | Stenosis in the Subclavian, Axillary, Brachial arteries |
| High distal arterial resistance | Inherent radial or ulnar arterial disease such as peripheral vascular disease, raynaud’s phenomenon, systemic sclerosis.-Diabetes Mellitus (DM) is a risk factor-Prior instrumentation of the artery such as arterial line placement leading to an atretic vessel |
| Low venous outflow resistance | Artery-to-vein/Artery-to-graft anastomosis made too large. -Retrograde flow from ‘high-resistance’ arterial bed (distal arteries) into the ‘low-resistance’ venous system (AV access) -DM is a risk factor for DASS as it causes impaired adaptation of inflow, outflow and collaterals, leading to a complex hemodynamic imbalance that causes DASS |
DASS – Onset, Signs and Symptoms
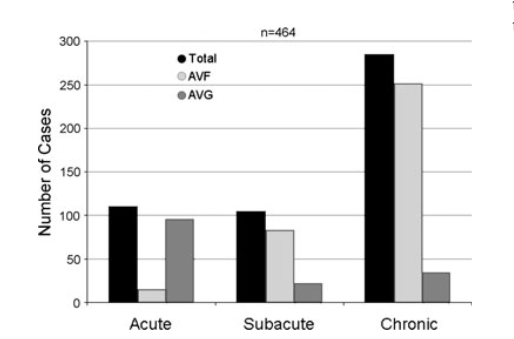
The onset of DASS symptoms after AV access creation tends to be more acute after AV graft (AVG) creation compared to AV fistula (AVF) in which the onset of DASS symptoms are generally subacute to chronic in onset
| Stage I | Retrograde diastolic flow without any symptoms |
| Stage II | Pain during exercise and/or during dialysis |
| Stage III | Pain at rest |
| Stage IV | Tissue loss: Ulceration/necrosis/gangrene |
Some other signs and symptoms associated with DASS are:
-Diminished or absent radial and ulnar pulses
-Relief of symptoms on compression of AV access
-Brachial artery digital index of 0.6 or less
-Digital pressures below 50 mmHG or non-recordable-Abnormal doppler waveform
Acute DASS symptoms include coolness, pallor, pain, tingling, and numbness in the distal extremity, whereas chronic DASS symptoms are due to chronic hypoperfusion of the distal extremity and include nail changes, ulcer, gangrene, muscle/tissue atrophy
Additionally, a rare but potentially devastating complication of DASS is ischemic monomelic neuropathy (IMN). IMN is a variant of DASS caused due to an ischemic nerve damage leading to severe sensorimotor dysfunction of the ulnar, median and radial nerves without evidence of peripheral soft tissue damage. The onset of IMN is immediate after AV access creation, and is associated with excruciating hand pain. Early recognition of IMN is crucial as it requires AV access ligation
Testing and Diagnosis
If the clinical symptoms and physical examination are suggestive of DASS, then the AV access flow and digital BP are measured using Doppler ultrasonography with and without AV access compression. Relief of symptoms on compression of the AV access is highly suggestive of DASS, as occlusion of flow in the AV access improves distal perfusion.
Treatment of DASS
Medical intervention is dependent on the stage of DASS
Patients deemed candidates for surgical intervention must undergo angiography to assess for presence of arterial stenosis proximal as well as distal to the AV access. Angioplasty of arterial stenosis can restore arterial perfusion and potentially relieve DASS symptoms
1. Arterial inflow
Angiographic imaging must include the entire arterial tree that supplies blood to AV access, including subclavian artery from its origin at the aorta, the inflow artery, the AV anastomotic site. Any stenotic lesions can be intervened on with the help of balloon angioplasty.
2. Distal arterial flow
One should make sure that distal artery is assessed for presence for stenosis. Restoration of distal arterial flow with AV access compression supports the diagnosis of DASS
If restoration of arterial inflow and distal artery arterial perfusion does not relieve the symptoms of DASS then one has to choose a surgical option that is best suited for the patient
- DRIL (Distal Revascularization – Interval Ligation)
- PAI (Proximalization of Arterial Inflow)
- RUDI (Revision using Distal Inflow)
- Ligation of the AV access
- Banding of the inflow
As shown above, DRIL consists of a low resistance collateral conduit (either reversed saphenous vein graft or PTFE graft) anastomosed from proximal inflow artery to distal outflow artery resulting in greater perfusion to hands/fingers. To prevent retrograde flow in diastole the artery below the AV access is ligated (abolishes the ‘suction effect’ of blood through the AV access from distal artery). Blood is still able to flow from the inflow to the outflow vein as it is a low resistance pathway.
The PAI procedure involves using a PTFE graft to create a more proximal AV anastomosis resulting in recruitment of more collaterals which could not be recruited earlier due to the more distal nature of the AV anastomosis. This enhances perfusion to hands/fingers and also removes the need to ligate the artery below the original AV access, making it a less invasive process.
The RUDI procedure is particularly useful in steal syndrome related to high flow brachial AV access. The anastomosis is closed and an interposition graft is placed between the outflow vein and a distal ulnar or radial artery. This reduces access flow rate by as much as 50% (Minion et. al). This procedure is an alternative to banding but should be used only if distal artery used for ‘new’ inflow is patent, otherwise there is a high risk of persistent steal. An advantage of this procedure is it lengthens the needling area for dialysis access.
Banding
This aims at reducing access flow, thus can be performed only in patients with high flow related DASS. It can be done with non absorbable sutures, small interposition graft, or by narrowing the vessel with a tight Dacron or PTFE cuff. Banding a low flow access can resolve steal but will lead to inadequate dialysis and raise potential for access thrombosis. When banding is done under intraoperative flow measurements with goal of ~400ml/min in native fistula and ~600ml/min in grafts, post-operative access patency and dialysis adequacy are better.
Ligation
Ligation resolves steal syndrome immediately. However it will lead to loss of dialysis access and cause the need for alternative access creation which again will be at risk of steal syndrome. Ligation is indicated if there is acute limb ischemia or IMN as described above. Reactivation of the access may be attempted but there is a high risk of thrombosis once ligated. A proposed algorithm in literature is to ligate the access, diagnose the etiology, perform DRIL, RUDI or PAI then reactivate the fistula.
A Word on Prevention
Prevention is the key. It is crucial that vessel mapping is done prior to the creation of the AV access. Careful physical examination and duplex imaging should be done to assess for arterial insufficiency. If arterial stenosis is identified then angioplasty must be done prior to AV access creation. In high risk patients, the following must be considered:
–Avoid distal brachial artery inflow
–Limit the size of the arterial anastomosis to 4 mm
–Consider pre-emptive RUDI if proximal radial/ ulnar arteries are suitable
–Consider pre-emptive PAI
Bhavnish Bucktowarsing, MD
ASDIN fellow
Acknowledgments: This post is part of a collaboration between the Renal Fellow Network and the American Society of Diagnostic and Interventional Nephrology (ASDIN), whose mission is to provide excellence in dialysis access care to improve outcomes for patients with kidney disease. Special thanks to Tushar Vachharajani, Aisha Shaikh, Edgar Lerma, and the Education Committee of ASDIN for their comments and suggestions for this post. For more information about the ASDIN mission or membership, click here. We would also like to thank Anil Agarwal, Robin Shah, Nabil Haddad, Khaled Boobes for assisting with this post.
Please note: these cases are vignettes created for educational purposes and patient consent has been obtained by author for clinical images.



Very well presented
This is absolutely one of the best-conceived, best-organized, and best-executed presentations I have encountered in any medical field. You have presented a complex and subtle pathophysiology and its treatment in a way that any doctor should be able to understand, and you have done so without sacrificing accuracy, depth, or important points (IMN, rôle of initial choice of site).
I so frequently encounter residents who have been beaten into near-hopeless confusion — just as I was — by years of needlessly complicated and brutally uninformative teaching on topics which are really much simpler than this one (basic ventilator modes, thromboelastography, etc.).
Thanks for giving me a little bit of hope for the future of our profession.
Georg Herlitz, MD, JD
Nice one Bhav! Impressive abstract.
Nice & informative
nice