As nephrologists, we remember the not so distant past when there were few options available to help patients battle the debilitating effects of anemia of chronic kidney disease. Fortunately, advances over the past two decades have led to the use of erythropoietin stimulating agents (ESAs) as the standard of care for this disease. The nephrologist is now tasked with not only the proper dosing of these agents but also with balancing the benefits against potential adverse effects. Here, we will review the four major trials which illustrate the history of ESAs (NHCT, CREATE, CHOIR, and TREAT), along with a discussion of the newer strategies on the horizon for managing anemia of chronic kidney disease.
The United States Normal Hematocrit Cardiac Trial (NHCT)
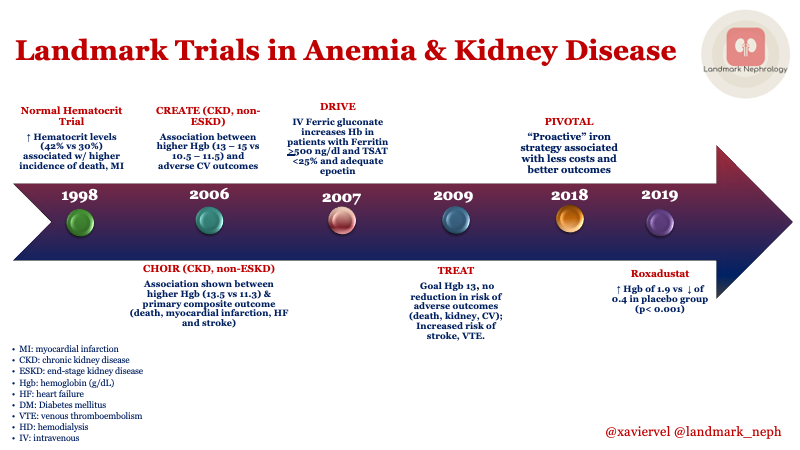
In 1998, The New England Journal of Medicine published the NHCT study which evaluated the effects of using Epoetin (recombinant human erythropoietin) to increase or “normalize” hematocrit to 42% (as compared to 30%) in hemodialysis patients. Based on prior studies, increasing hematocrit levels were associated with positive cardiac measures and a better quality of life. Somewhat unexpectedly, the results of the NHCT revealed a trend of higher mortality and non-fatal myocardial infarction in the normal-hematocrit group, resulting in halting the trial early due to these findings. Check out this NephJC post on correction of anemia and quality of life,
Correction of Anemia with Epoetin Alfa in CKD (CHOIR)
Almost a decade later, the CHOIR trial assessed patients with CKD (not on dialysis) to determine if targeting higher hemoglobin levels (>13.5 g/dL vs. >11.3 g/dL) would have beneficial effects on the rate of cardiovascular events. The study enrolled over 1,400 patients with a median study duration of 16 months. The authors here found no clinical improvement in the quality of life between the two groups but alarmingly an association between higher hemoglobin with the primary composite outcomes (death, myocardial infarction, and hospitalization for heart failure and stroke).
Normalization of Hemoglobin Level in Patients with CKD and Anemia (CREATE)
The CREATE trial, published along with CHOIR, also examined the treatment of anemia in patients with CKD (not on dialysis). Here, patients were randomized to normal hemoglobin (13-15 g/dL) and compared with hemoglobin levels in the range of 10.5-11.5 g/dL over a three year study period. Overall the group with higher hemoglobin levels showed improved general health and physical function, however they showed increased headaches and hypertensive episodes.
Findings were consistent with CHOIR, revealing an association between normal hemoglobin levels and adverse outcomes such as increased cardiovascular events, dialysis initiation, hypertensive episodes, and thrombotic events.
What followed the publication of some of the aforementioned trials deserve a post on its own, luckily for us Dr. Mark Rosenberg and Dr. Daniel Coyne have succinctly summarized one of the most famous missteps in nephrology, such as the story of Normalization of Hemoglobin, which was heavily featured in NephMadness 2016.
A Trial of Darbepoetin Alfa in Type 2 Diabetes Mellitus and CKD (TREAT)
The TREAT trial was the final nail in the coffin for normalization of hemoglobin. This particular trial had major differences with the aforementioned trials. TREAT was the largest trial to date with 4,038 patients and examined patients with CKD and type 2 diabetes (not on dialysis). It was double-blinded and compared treatment of darbepoetin alfa with target hemoglobin goal of 13 g/dL against placebo with a median follow up of 29.1 months. The conclusion was that the use of darbepoetin alfa did not reduce the risk of either death, cardiovascular, or a kidney event. However, it was associated with an increased risk of stroke in a subgroup analysis.
Now let’s fast forward to what new therapies for anemia in CKD could be coming in the future …
Intravenous Iron in Patients Undergoing Maintenance Dialysis
Two trials deserve mention, the first one, is the DRIVE trial published in 2007. This was an open-label, randomized, multicentric trial across 37 sites in the United States; 134 patients were randomized to receive 125 mg of ferric gluconate versus no iron. Patients were receiving maintenance dialysis and had to have ferritin > 500 mg/dl and TSAT <25% while getting adequate doses of epoetin. Hemoglobin rose faster and higher in the intervention group, demonstrating that the supplementation of intravenous iron was capable of surpass the anemia associated to iron deficiency and inflammation.
The PIVOTAL trial, published in early 2019, compared the effect of proactive vs reactive IV iron administration on the number of cardiovascular events, deaths, transfusions and dose of erythropoiesis-stimulating agents in patients with ESKD on maintenance hemodialysis. Prior to this trial, the use of IV iron had not been studied in large controlled trials. A total of 2,141 participants were randomized 1:1 to receive either a reactive strategy of low dose of intravenous iron sucrose versus a proactive strategy of high dose of intravenous iron sucrose.
During the first year of the trial, the group receiving proactive IV iron had a rapid improvement of their anemia with hemoglobin values averaging 11.2 g/dL versus 10.6 g/dL. The proactive iron strategy patients received less cumulative ESAs at all post baseline time points, thus leading to a potential benefit via decrease in total treatment costs. Ultimately, the proactive IV iron therapy also reduced the incidence of the primary end point – a composite of first non-fatal myocardial infarction, nonfatal stroke, heart failure hospitalization or death.
The Dawn of the HIF Pathway
The discovery of the “oxygen sensing pathway” that led to the 2019 Nobel prize in physiology and medicine is the basis of our next study.
Hypoxia-inducible factor (HIF) has been identified as one of the key regulators that control how cells respond to hypoxic conditions. It enhances kidney and hepatic EPO synthesis; it also enhances iron uptake by the intestine and opposes the deleterious effects of hepcidin (implicated in the pathophysiology of EPO resistant anemia). A prolyl-hydroxylase inhibitor (PHI) stabilizes HIF and prevents its degradation, allowing the stimulation of EPO gene expression in the kidneys.
Roxadustat is one of several oral HIF-PHI enzymes to enter the arena of treatment for patients with anemia of CKD. A phase 3 trial published in the NEJM in September 2019 examined 154 non-dialysis, CKD patients in China. These patients were randomly assigned (2:1) to receive Roxadustat or placebo in a double-blinded fashion. The group that received the HIF inhibitor saw a change from baseline in the hemoglobin level, with an average increase of 1.9 g/dL versus a decrease of 0.4 g/dL in the placebo group (P < 0.001). Roxadustat was not devoid of side effects however, which warrants a careful look at the results. Specifically, patients in the treatment group experienced more hyperkalemia and metabolic acidosis. Despite these results, we eagerly await results of ongoing studies in patients on dialysis.
In conclusion, the treatment of anemia in our kidney disease patients can now be summarized into three aspects of management: First, high hemoglobin levels were not beneficial based on the early trials with ESAs; second, utilization of a more proactive approach with intravenous iron could be beneficial; and lastly, the excitement around HIF-PHI agents represents potentially new horizons.
Post by: Xavier Vela
Landmark Nephrology is an online learning tool designed to collect landmark trials in nephrology and distribute content that makes learning nephrology fun and easy.
Please visit us to check out our topic-specific content including videos, visual abstracts, quizzes, and a new slide-share portal to facilitate the exchange of educational material within the nephrology community.
We love collaboration so contact us as landmarknephrology@gmail.com or find us on Twitter to get involved!


GOOD, summarized review.
I am a pediatric nephrology fellow