Welcome to the 18th case of the Skeleton Key Group, a team of 50-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network. The cases are actual cases (without patient identifying information) that intrigued the treating fellow.
Written by: Raad Chowdhury, MD
Visual Abstract & Infographics: Carlo Trinidad, MD, Brian Rifkin, MD, FASN , Paresh Jadav, MD, FASN
A. The Stem
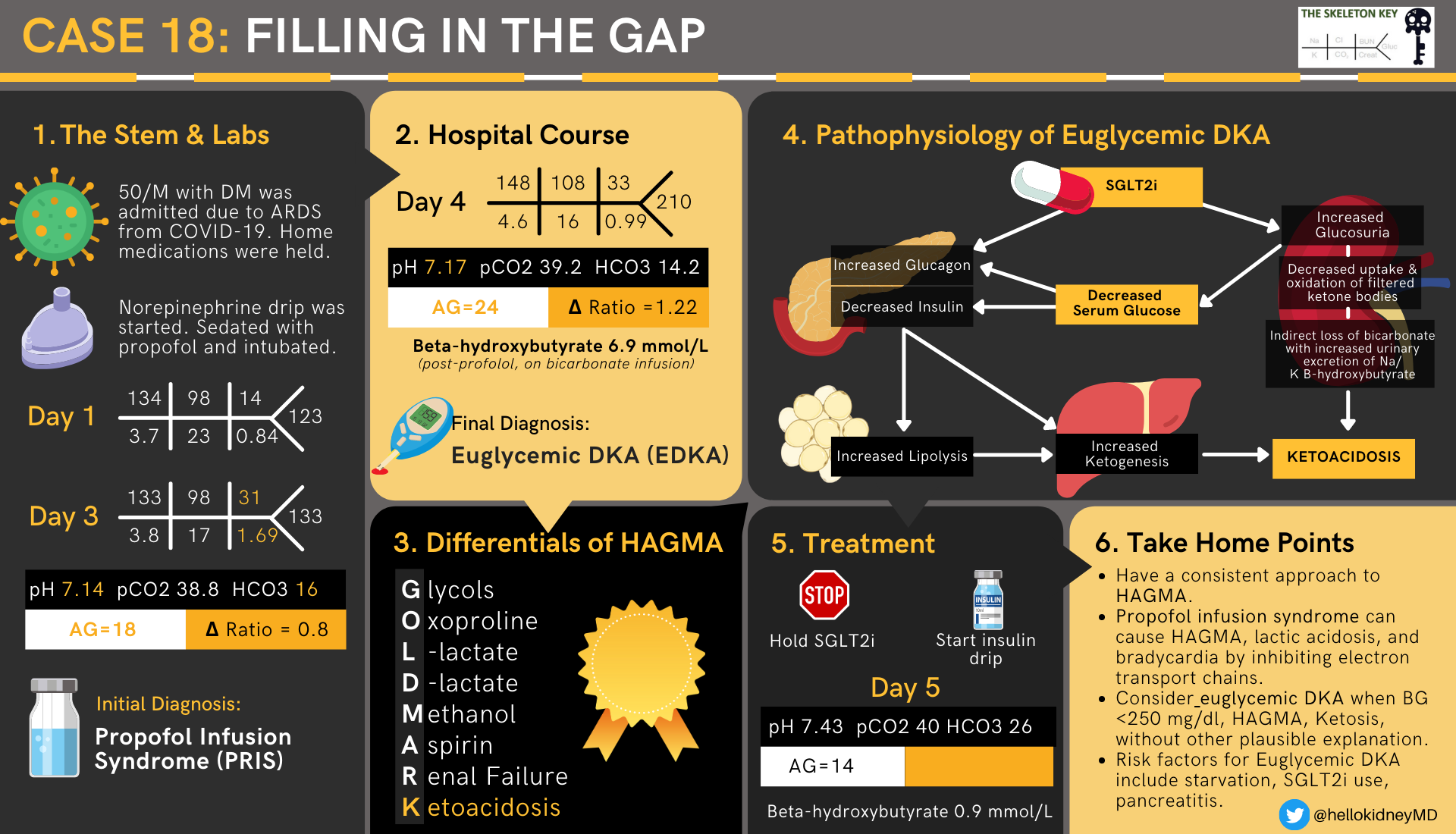
A 50-year-old man with diabetes mellitus on oral medications presented with respiratory distress and was found to be positive for SARS-CoV-2, requiring prompt intubation in the emergency department. Computed tomography (CT) of his chest showed bilateral ground glass opacities suggestive of acute respiratory distress syndrome (ARDS) and he was admitted to the MICU. He required norepinephrine and vasopressin for blood pressure support, and propofol for sedation. At the time of admission, all home oral medications were held.
On hospital day 3, nephrology was consulted for metabolic acidosis and acute kidney injury (AKI).
B. The Labs
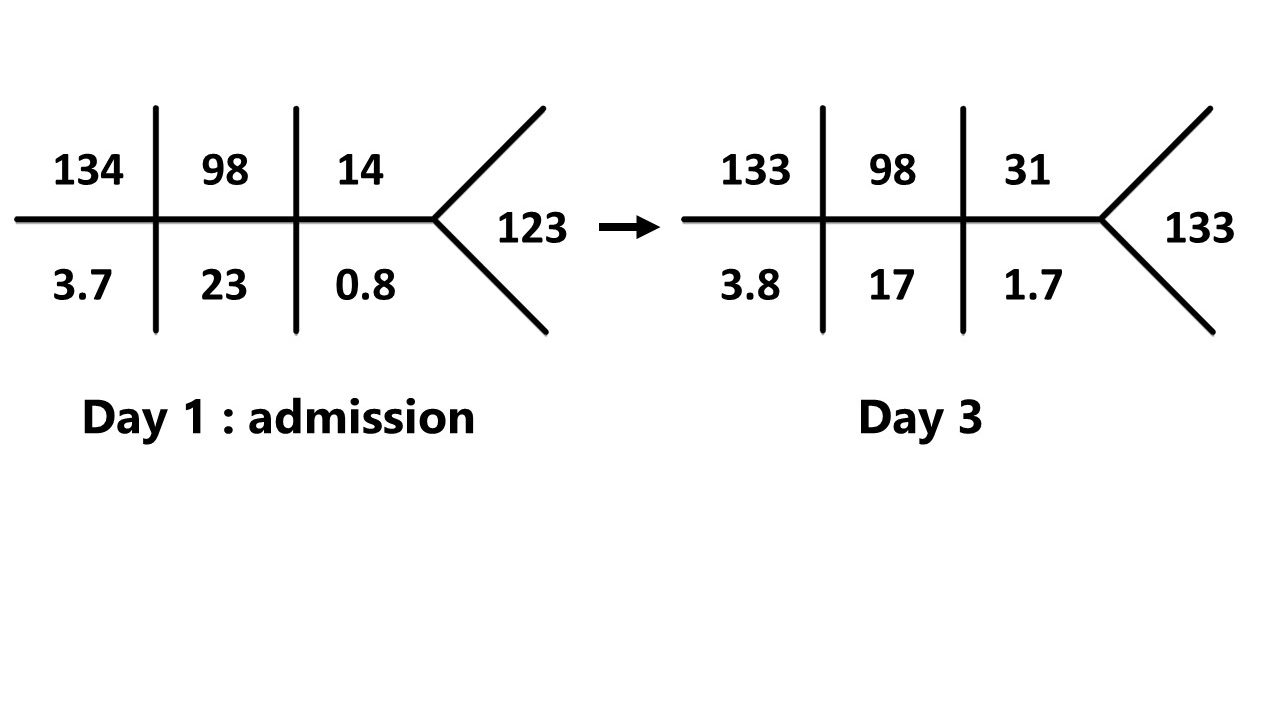
What are the lab abnormalities noted?
Patient developed worsening metabolic acidosis and new onset AKI. We hypothesize the AKI is due to hemodynamic instability requiring pressor support.
What do you order next?
ABG:
pH 7.14
pCO2 39 mmHg
pO2 80 mmHg
Bicarbonate: 16 mmol/L
Stop: What is the acid base disorder?
Here is our approach:
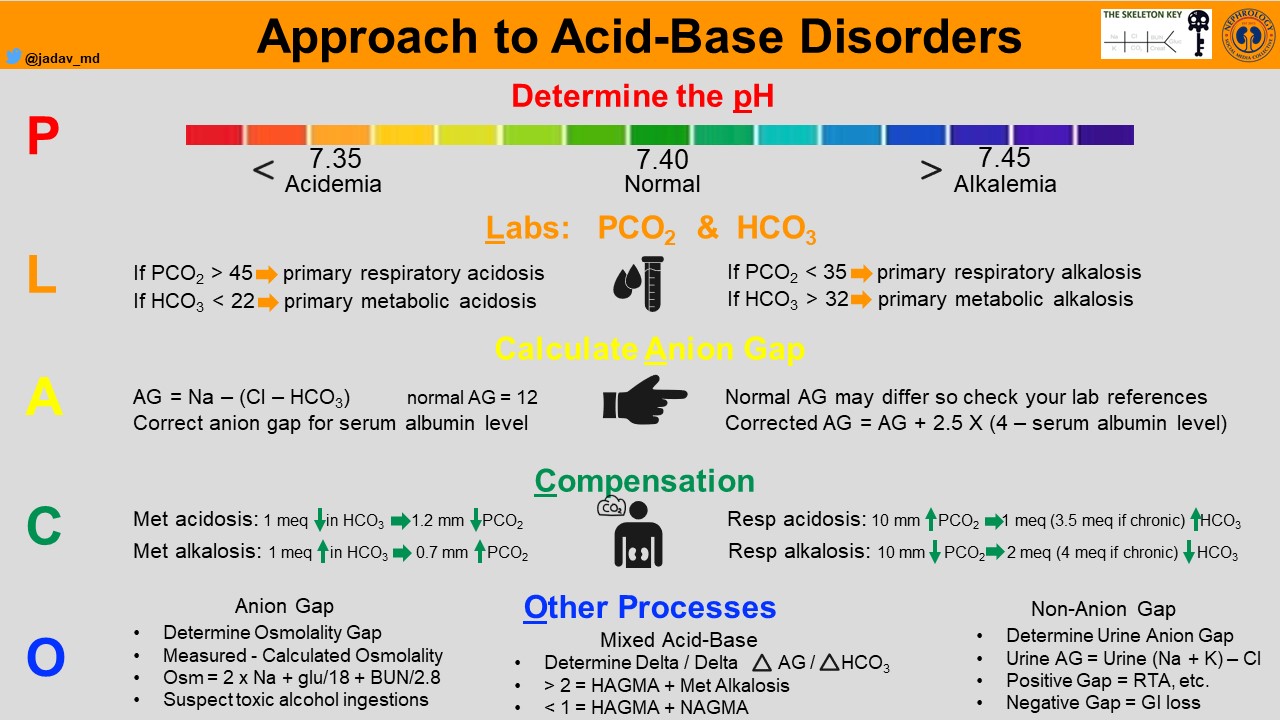
1) Evaluate the pH to identify if acidemia or alkalemia exists.
*Teaching point:
Acidemia is the blood pH less than 7.35. Acidosis is the physiological process occuring that lowers the pH by increasing H+ ions.
Acidemia: pH 7.14
2) Evaluate the pCO2 to determine if respiratory or metabolic.
normal pCO2 (39) and Low HCO3– (16). Remember a high pCO2 would have been suggestive of a primary respiratory acidosis.
STOP: Primary Metabolic Acidosis
3) Evaluate for appropriate respiratory compensation using Winter’s formula and bicarbonate from ABG. Expected pCO2 = [(1.5 x HCO3–) + 8 ] +/- 2
STOP: Expected pCO2 ~29 +/- 2. The patient’s actual pCO2 was 39, suggesting a concomitant respiratory acidosis.
4) Calculate Anion Gap (AG) = Na – (Cl + HCO3–)
AG= 18 (normal AG=12), Therefore a high AG metabolic acidosis (HAGMA) exists.
5) Calculate the delta ratio using the chemistry panel above (see below graphic): ΔAG/ΔHCO3– (18-12)/(24-16) = 0.8
STOP: A delta ratio 0.9 signifies a pure HAGMA In general, a ratio of 0.8-2 usually signifies a pure HAGMA. A ratio >2 signifies a metabolic acidosis with concomitant metabolic alkalosis.

Final interpretation: HAGMA with respiratory acidosis. The respiratory acidosis is likely due to permissive hypercapnia in managing ARDS and sedation. The metabolic component could be explained by new onset AKI but the degree of acidemia was quite significant and other alternative causes were sought.
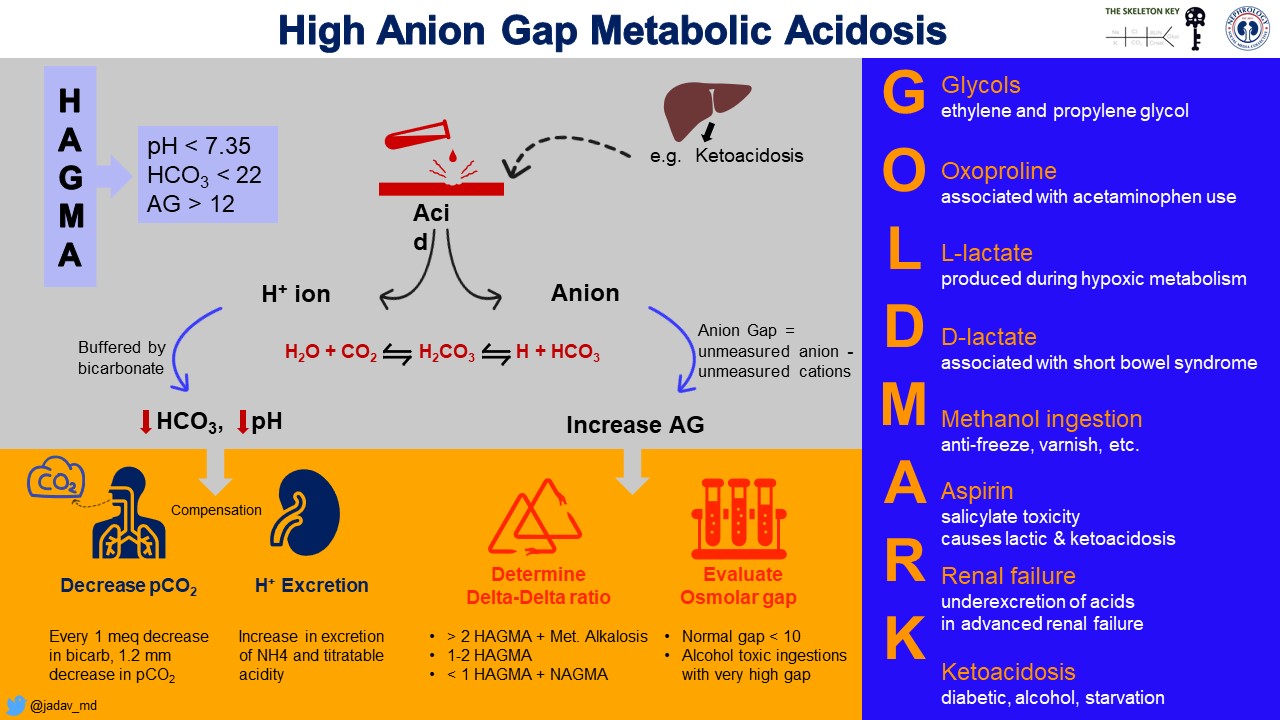
C. What are the differentials for HAGMA?
Glycols (ethylene, propylene)
Oxoproline
Lactic acidosis
D-lactic acidosis
Methanol
Aspirin
Renal Failure – sulfate, phosphate
Ketoacidosis
Propofol Infusion Syndrome
Physiology Alert #1: Before we proceed further, what truly is an Anion Gap?
The concept of an “anion gap” was first introduced in 1939 using a visual description called a “Gamblegram” devised by physiologist James Gamble. The idea was to demonstrate the law of electroneutrality which states that in plasma, the charge of cations (e.g., Na+, K+) must equal the charge of anions (e.g, Cl–, HCO3–). Routine current laboratory techniques are unable to explicitly quantify all the extra anions that may exist during disease states. Examples of these unmeasured anions include sulfate and phosphate derivatives, ketoacids, formic acid, and glycolic acid, just to name a few. Many of these anions are breakdown products of toxic ingestions that are buffered by bicarbonate. Thus, an increase in these unmeasured derivatives can tilt the electroneutrality towards anions. The anion gap (AG) estimates the amount of unmeasured anions. It has a normal range between 8-12, which may vary between labs. When the levels of these unmeasured anions exceed those of the unmeasured cations, an anion gap will result.
Physiology Summary #1:
1) AG assesses unmeasured anions that the lab cannot routinely identify.
2) When levels of unmeasured anions exceed those of unmeasured cations, AG will result.
There are two main outcomes that can occur:
1) High AG acidosis as described above.
2) Normal or low AG acidosis.
Albumin: The majority (80%) of the unmeasured anions in the circulation (remember the 12 number) are due to the presence of circulating proteins (Kraut et al CJASN 2007). Because albumin is the most abundant protein circulating in the blood, reductions in albumin leads to a reduction in the AG. In fact, for every 1 g/dl decrease in serum albumin, the serum AG decreases by 2.5 mEq/L. So it is imperative to know the serum albumin concentration. Thus, if a patient with serum albumin of 1 has an AG of 12, this is actually abnormal and indicative of a positive AG.
Hypergammaglobulinemia: In rare instances elevated Immunoglobulin G (IgG), which is cationic, can cause a low or even negative anion gap. Interestingly, IgG concentrations are inversely correlated with AG, therefore the highest concentrations of IgG tend to have low or even a negative AG. In contrast, IgA is anionic and can increase the gap.
Back to the Case: The three possibilities that exist with current data are
- propofol infusion syndrome: patient requiring high doses of propofol.
- renal failure: patient accumulating sulfates and phosphate based acids.
- ketoacidosis
At this juncture his lactate level is 2.0, beta hydroxybutyrate was 2.9 mmol/l (normal <0.5 mmol/l), blood sugar was 131 mg/dl. There was low suspicion for ingestion but a volatile panel to detect and quantify toxic alcohols were sent.
C. Hypothesis #1
Given the current data at hand we hypothesized the following:
The respiratory acidosis is likely due to the ongoing SARS-CoV-2 infection and COVID-19.
We felt the AG metabolic acidosis may be driven by the high propofol infusion rates the patient was requiring (>4mg/kh/hr), manifesting as propofol infusion syndrome (PRIS); however, the timing and lack of other features of PRIS make the diagnosis less likely. Nonetheless, the decision was made to wean the patient off propofol and start a bicarbonate infusion.
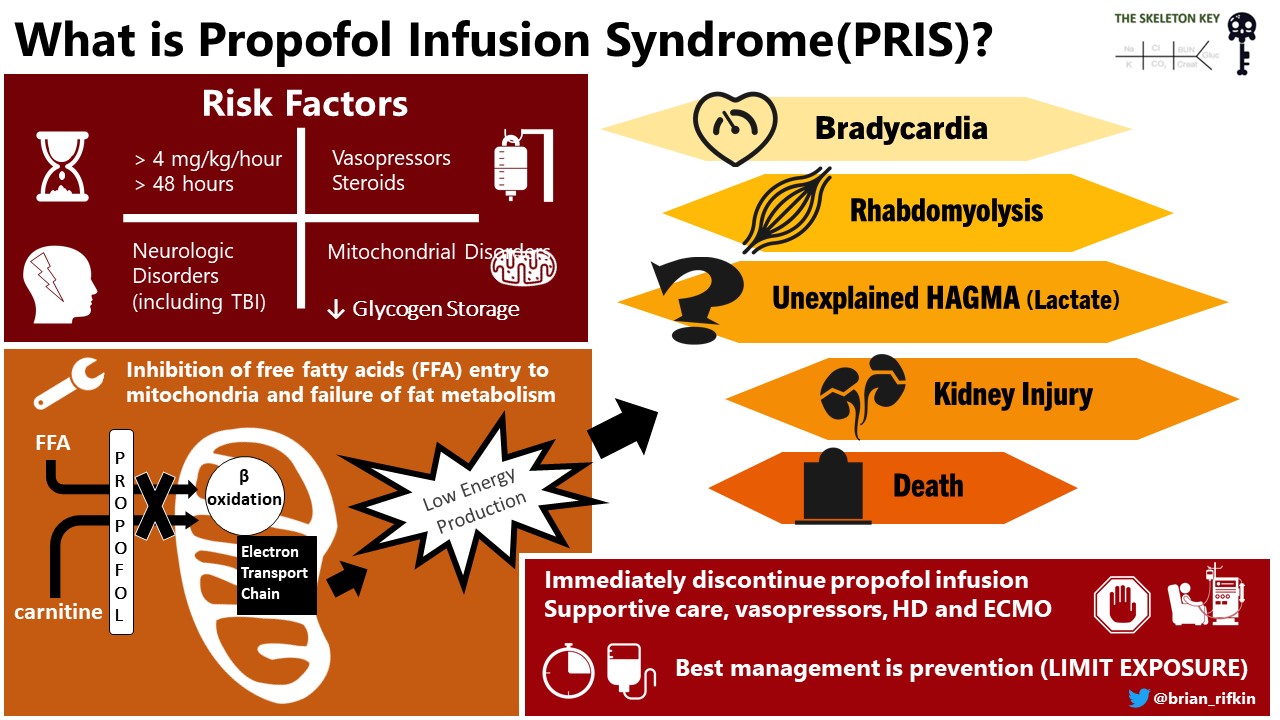
What is Propofol Infusion Syndrome (PRIS)?
Propofol infusion syndrome is an extremely rare and fatal constellation of findings that occur in patients receiving high doses of propofol regardless of duration. Doses >4mg/kg/hr for more than 48 hours are all risk factors for PRIS, which our patient was requiring. Some of the other deleterious effects include bradycardia, rhabdomyolysis, metabolic acidosis, kidney failure, and even death(Mike, Practical Gastroenterology 2010).
Physiology Alert #2
The proposed mechanism of this unexplained acidosis is through propofol’s effect on the electron transport chain in mitochondria. In animal models, it is believed that propofol interferes with oxidative phosphorylation which therefore would prevent generation of electrons, protons, and formation of H2O. This would ultimately cause a failure of the electron transport chain (ETC) and generation of ATP would decrease. Decreased ATP would lead to increased glycolysis and increased production of pyruvate, leading to formation of lactate(Koenig, Pediatric Neurology 2008). However, our patient had a normal lactate on multiple checks.
Propofol ⇒ Impairs ETC ⇒ Decreased ATP ⇒ Increased Pyruvate ⇒ Increased lactate
D. More Data
Hospital Day 4 (off propofol, on bicarbonate infusion 150 meq/L sterile water @ 250 ml/hr)
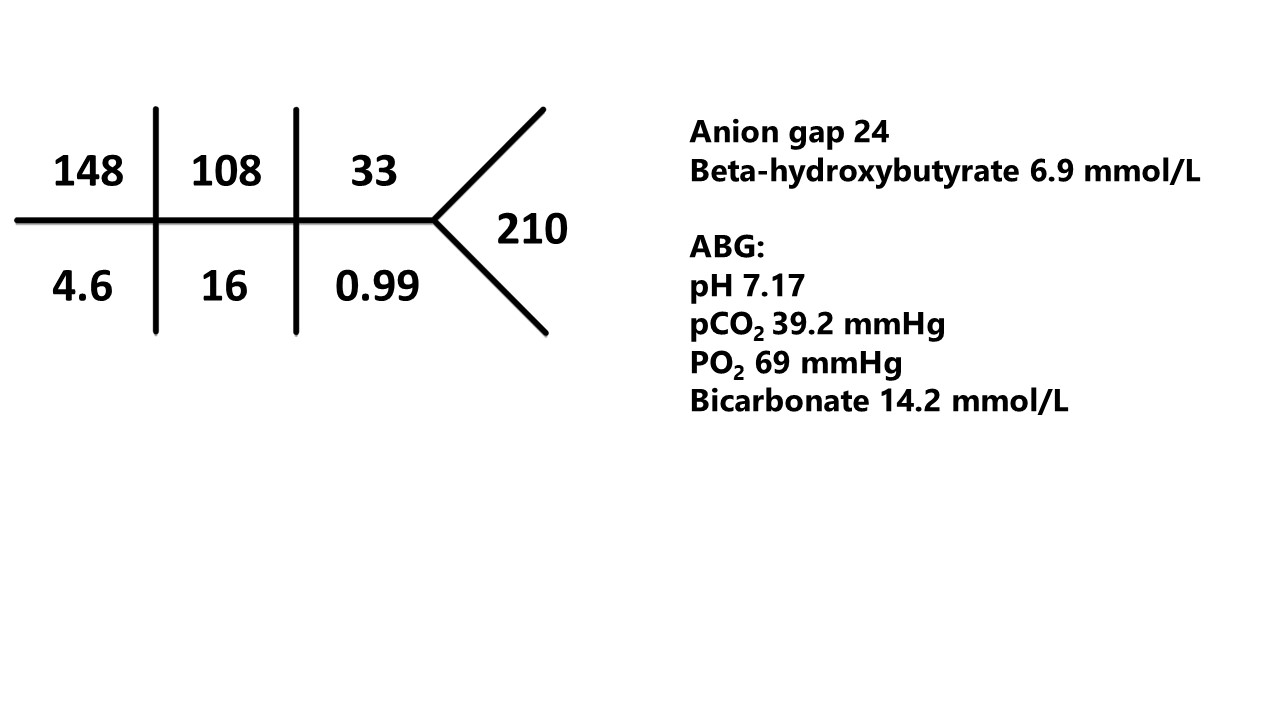
What has changed?
- AG is wider at 24 (was 18) .
- Bicarbonate is lower while on the bicarbonate infusion and after stopping propofol (was 17 on day 3 chemistry and 16 on initial ABG).
- Blood sugar is higher.
- Beta hydroxybutyrate is higher (2.9 to 6.9)
Stop: What is the acid base disorder?
The patient now has a pure high anion gap metabolic acidosis and respiratory acidosis.
- This is because the AG is 24
- The expected pCO2 using winter’s formula is ~29 mmhg. Actual pCO2 is 39 mmhg.
- The delta ratio is 12/10 = 1.2. Recall that a delta ratio 1-2 is usually a pure HAGMA.
The only possibilities that exist with current data: ketoacidosis: BHB now increased to 6 from 2.9 mmol/l, BG 180-210 mg/dl
Hypothesis 2
The AG is wider with an increase in BHB. Blood glucose is now 210 mg/dl (11.7 mmol/l). Propofol has been off for 24 hours and the bicarbonate drip seems to have improved the non-anion gap component but treatment requires addressing the underlying cause. It was also determined that he was on an SGLT2 inhibitor at home which was stopped on admission. This could represent euglycemic diabetic ketoacidosis (EDKA) and an insulin drip was started.
Hospital Day 5 (post Insulin Infusion with Insulin Drip)
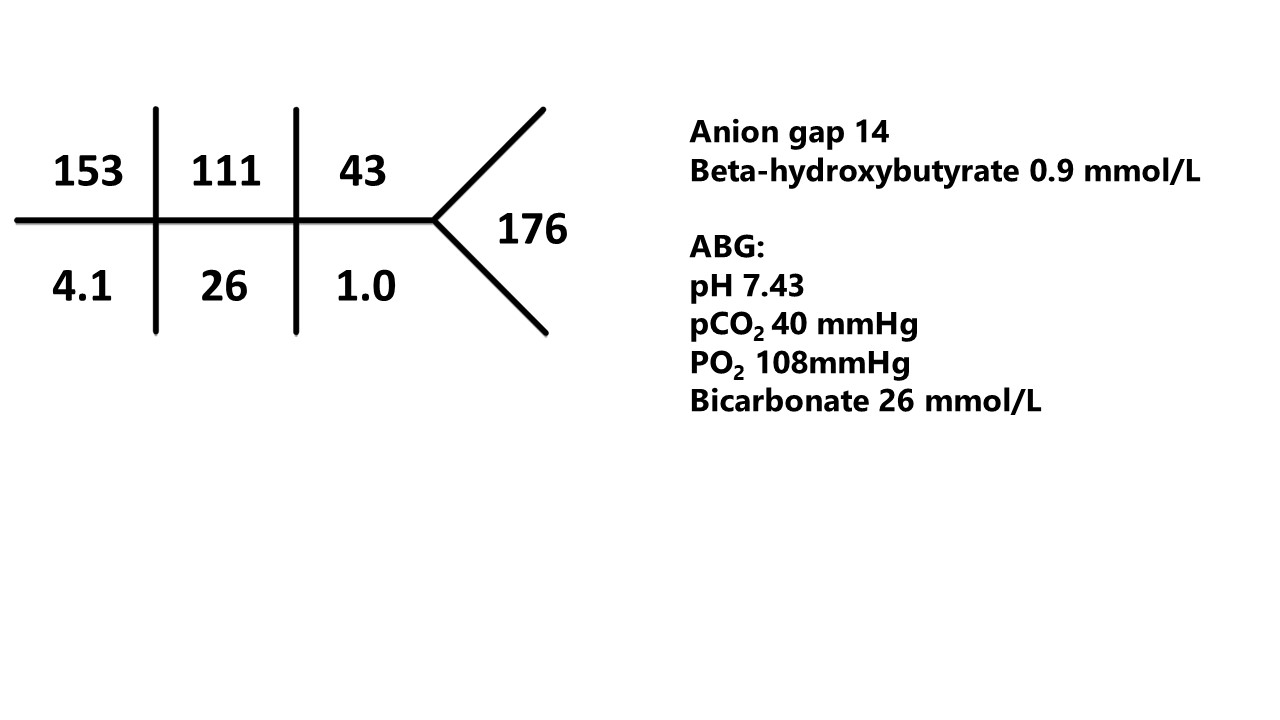
E. Final Diagnosis: Euglycemic DKA (EDKA)
According to the American Diabetes Association, conventional DKA is defined as hyperglycemia (BG >250 mg/dl or 13.9 mmol/l), acidosis (pH <7.3, bicarbonate <15), and ketosis. However, in 1973, Munro et al. described an unusual phenomenon of ketoacidosis with normoglycemia after review of 211 cases. They found that in those cases, 37 had blood glucose less than 300 mg/dl with unexplained anion gap metabolic acidosis and ketonemia. Since then, there is increased appreciation that a subset of patients do indeed have normoglycemia as defined by BG <250 mg/dl and still manifest other characteristics of DKA, which became known as EDKA. The current accepted definition of EDKA is blood glucose less than 250mg/dl, anion gap metabolic acidosis, ketonemia and ketonuria. Possible causes of euglycemic DKA include SGLT2i, insulin administration prior to coming to the hospital, concurrent food restriction, vomiting, and other inhibition of gluconeogenesis. Interestingly, the half life of SGLT2 inhibitors range from 12-13 hours and our patient developed obvious hyperglycemia with ketonemia 2-3 days post hospitalization. Recently, Five cases of EDKA with COVID-19 have been reported. All the patients had diabetes treated with SGLT2 inhibitors, were diagnosed with COVID-19, and subsequently developed EDKA. SGLT2 inhibitors were held on admission and patients were treated with insulin infusion similar to our case (Vitale et al. AACE 2021). It remains unclear as to why EDKA develops in this particular situation even when being held on admission.
Pathophysiology Alert #3
The pathophysiology of EDKA is believed to be from decreased serum glucose stimulating glucagon, epinephrine, and cortisol, while suppressing insulin. Increased glucagon and decreased insulin stimulates lipolysis and formation of free fatty acids leading to beta-oxidation and the formation of ketones.
Pathophysiology Alert #4
How do SGLT2is work in the kidneys?
- Inhibits the sodium glucose cotransporter-2 S1 segment of the early proximal tubule. This is where over 80% of the glucose is reabsorbed and inhibition of this transporter leads to glucosuria and decreased plasma glucose concentration.
How do SGLT2 inhibitors cause ketoacidosis?
- Inhibits SGLT2 receptors in the pancreatic alpha-cells. This leads to glucagon secretion. Glucagon suppresses insulin decreasing carbohydrate metabolism and increased fatty acid metabolism. This eventually forces the liver to produce ketones.
- Interferes with proximal tubule ATP consumption. Once the sodium coupled glucose transporter is inhibited, less Na is reabsorbed by the proximal tubule which also decreases ATP consumption. Decreased ATP consumption leads to decreased ammoniagenesis, bicarbonate loss, and fat oxidation by the kidney.
- Increases fat oxidation in the proximal tubule. This further suppresses ammoniagenesis and exacerbates bicarbonate loss.
TAKE HOME POINTS
- GOLDMARK
- Have a consistent approach to HAGMA.
- Propofol infusion syndrome can cause HAGMA, lactic acidosis, and bradycardia by inhibiting electron transport chains.
- Consider euglycemic DKA when BG <250 mg/dl, HAGMA, ketosis, without other plausible explanation.
- Risk factors for euglycemic DKA include starvation, SGLT2 inhibitor use, and pancreatitis
Reviewed by Matthew A. Sparks, Joel Topf, Chi Chu, Alejandro Meraz, Dominique Tomacruz, Jamie Willows, Anna Gaddy Sayna Norouzi, MD


