Welcome to the 29th case of the Skeleton Key Group, a team of 50-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network.
Written by: Alessandra Rader Visual Abstract & Infographics: Sharanya Ramesh
A. The Stem:
A 54-year-old man with alcohol-associated cirrhosis presented to the hospital with several days of abdominal distention. He was requiring weekly paracentesis for ascites. However, this time he reported more abdominal distention than usual, and was drinking much more water. He was adherent to his home medications, including furosemide, spironolactone, and propranolol. He denied nausea, vomiting, or diarrhea. He had regular bowel movements without melena or hematochezia.
Vital Signs:
T: Afebrile; BP: 98/69 mm Hg; Pulse: 103 BPM; Resp: 18 breaths per a minute; SpO2: 99% on room air; Weight 70 kg
Physical Exam:
Gen: alert and oriented and in no acute distress
Heart: tachycardic, regular rhythm, no murmurs
Lungs: diminished basilar breath sounds bilaterally
Abdomen: non-tense, non-tender abdominal distention with a fluid wave
Extremities: trace peripheral edema
Neurological: intact cranial nerves, normal strength and sensation in all four extremities, no asterixis
B. The Labs:
Serum osmolality: 242 mOsm/kg
Urine studies:
Na: < 20 mmol/L
Cl: < 20 mmol/L
K: 32.3 mmol/L
Osmolality: 278 mOsm/kg
Uric acid: 6.1 mg/dL
AM cortisol level 5.9 mcg/dL
TSH: 0.52 mcg/dL
UA: pH 5.0; specific gravity 1.011 (reference range: 1.003-1.035); negative for protein, glucose, ketones, leukocyte esterase, nitrites, WBCs, RBCs, and casts.
Nephrology is consulted for hyponatremia.
C. Differential Diagnosis:
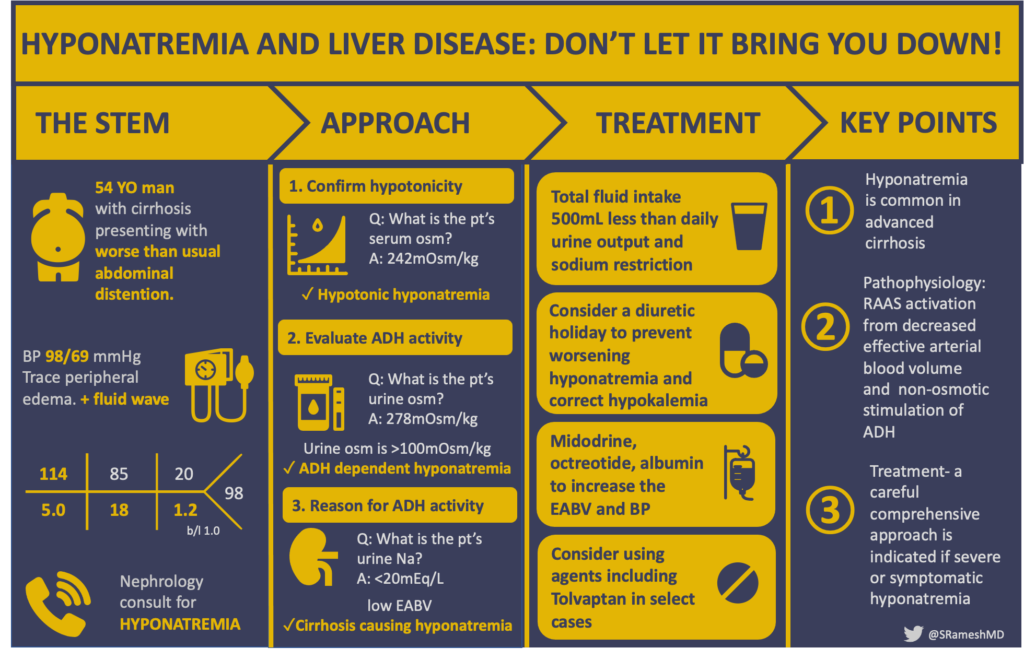
A framework to evaluate hyponatremia in cirrhosis.
Evaluating of hyponatremia
Let’s take a physiological approach in evaluating hyponatremia using the figure above.
FIRST: Confirm hypotonicity. This patient’s serum osm of 242 mOsm/kg is low (reference range 275-295 mOsm/kg) which confirms a hypotonic hyponatremia exists. If the serum osm > 275 this could be hypotonic, isotonic, or hypertonic and you would need to evaluate for effective vs ineffective osmoles contributing.
NEXT: Evaluate for the presence of antidiuretic hormone. This patient’s urine osmolality of 278 mOsm/kg is not maximally diluted (urine osmolality >100 mOsm/kg), which suggests an ADH-dependent hyponatremia.
FINALLY: Assess the underlying mechanisms driving ADH secretion in hypotonic hyponatremia—is the ADH secretion “appropriate” or “inappropriate” physiological? Low effective arterial blood volume is the primary “appropriate” driver of ADH secretion in hypotonic hyponatremia, and is characterized by activation of the renin-angiotensin-aldosterone system and increased sodium avidity in the kidneys (UNa <20 mEq/L). In this case, we excluded other causes of volume depletion (no reports of vomiting, diarrhea, or overdiuresis), leaving cirrhosis itself as the underlying cause of hypotonic, ADH-dependent hyponatremia.
Pathophysiology of Hyponatremia in Cirrhosis
The pathophysiology of hyponatremia in patients with cirrhosis is complex. It is theorized that intestinal-microorganisms and endotoxins bypass the portal circulation, enter the systemic circulation causing endotoxemia, leading to the synthesis of vasodilators like nitric oxide. Vasodilation (splanchnic > peripheral) and hyperdynamic circulation leads to activation of the renin-angiotensin system, increased sympathetic nervous system tone, and the nonosmotic release of ADH.
The pathophysiology of hyponatremia in cirrhosis involves portal hypertension, decreased renal blood flow, and activation the renin-angiotensin aldosterone system (RAAS) and sympathetic nervous system, and the non-osmotic release of antidiuretic hormone (ADH).
Prognostic implications of hyponatremia
Several studies have shown a correlation between hyponatremia and increased frequency of the major complications of cirrhosis. In a study of 997 hepatology clinic patients with cirrhosis and ascites, serum sodium concentrations < 135 mmol/L were associated with poor control of ascites and increased frequency of hepatic encephalopathy, hepatorenal syndrome, and spontaneous bacterial peritonitis when compared to patients with normal serum sodium concentrations. For patients with serum sodium concentrations < 130 mmol/L, there was an even greater frequency of severe ascites and associated complications. There was no association between low serum sodium levels and gastrointestinal bleeding. Moreover, several studies demonstrated that hyponatremia at the time of liver transplantation is associated with adverse outcomes post-transplantation. These include neurological complications, infectious complications, and kidney failure 30 days post-transplant and increased mortality 90 days post-transplant. As such, the MELD-Na, a score that reflects severity of liver disease, rises as the sodium falls below 140 mEq/L.
D: Management
Hyponatremia is common in cirrhosis and serves as a marker of circulatory and kidney dysfunction. Chronic, asymptomatic hyponatremia in cirrhosis does not require urgent treatment. Treatment is considered in severe or symptomatic hyponatremia. The following are considerations for the management of hypervolemic hyponatremia in cirrhosis:
Water and sodium restriction
In patients with hyponatremia related to cirrhosis, ADH levels are increased and the kidneys’ ability to eliminate solute-free water is impaired. This leads to a disproportionate accumulation of water in relation to sodium in the serum. As such, water and sodium restriction is a first-line treatment. The goal is to achieve a negative fluid balance. In order to be clinically effective, however, total daily fluid intake should be at least 500 mL less than the combined 24-hour urine output and insensible fluid losses. There are a few approaches to estimate this, as discussed in SKG Case #4. A formula to estimate urinary electrolyte-free water clearance is: Urine volume x [1 – (Urine Na + Urine K) / (Serum Na) ]. A rough estimate of urinary electrolyte-free water clearance is: ignore the first 1L urine, assume half of urine is free water for the next 1-3L urine, and assume all the urine beyond 3L is lost free water). However, fluid restriction in cirrhosis is often difficult to achieve due to patient comfort and thirst.
Correcting hypokalemia
Hypokalemia is also common in cirrhosis, often related to diuretics, laxatives, and the hyperaldosteronism associated with liver disease. Decreased total body potassium directly causes hyponatremia as shown in the Edelman Formula (If you haven’t spent time looking at this post on RFN, do this now!) As sodium and potassium are osmotically active and exchangeable, once potassium supplementation is provided it readily enters into cells, causing intracellular sodium to shift out and thus increase serum sodium levels. Additionally, hypokalemia is thought to worsen hepatic encephalopathy by increasing renal ammoniagenesis. Remember 1 mEq of potassium roughly equals 2 mL of 3% saline, so that 40 mEq bid of KCl you are ordering to fix the potassium is equivalent to a modest daily bolus of 3% saline.
Diuretics — to stop or to start?
Decompensated cirrhosis is often associated with secondary hyperaldosteronism, leading to the reabsorption of sodium and water in the distal convoluted tubules and collecting duct, contributing to the development of ascites and edema. To control these complications, aldosterone antagonists and loop diuretics are often initiated. In some cases, diuretics may worsen the hyponatremia by increasing sodium excretion. Some patients with worsening hyponatremia could benefit from a trial diuretic holiday. But on the other hand, stoppage of diuretics can lead to accumulation of extracellular fluid and may need to be reinstated. Finding the right balance is key.
Albumin infusion
Although the exact mechanism of action of albumin is unknown, it is hypothesized that it has both oncotic and non-oncotic properties. Firstly it increases effective arterial blood volume and secondly, reduces RAAS activity by increasing renal blood flow, ultimately resulting in increased free water excretion and decreased ADH levels. A recent multi-center study demonstrated that patients with cirrhosis and hyponatremia had greater resolution of hyponatremia when they received albumin infusions vs not. In addition, it was demonstrated that there was a positive correlation between the grams of albumin given and the change in sodium. Finally, the study found that patients who received albumin infusions had a better 30-day survival compared to those who did not receive albumin. However, this was an observational study that may be limited by residual confounding. There are no large randomized controlled trials examining the use of albumin in hyponatremia with cirrhosis.
Midodrine/Octreotide
Hypotension is a well-known complication of cirrhosis, mainly due to portal hypertension which leads to splanchnic and systemic vasodilation (as discussed above). In those patients who have persistent hypotension despite stopping diuretics and who have failed to improve with albumin infusion, midodrine and octreotide are considered to help support vascular circulation. Midodrine is an alpha agonist that promotes peripheral vasoconstriction, whereas octreotide is a somatostatin analog that acts by inhibiting the secretion of glucagon (a splanchnic vasodilator) and by direct mesenteric vasoconstriction.
Lactulose
The colon is another route in which electrolyte-free water can be removed from the body. Lactulose, a nonabsorbable substance in the colon, acts as an osmotic laxative. Essentially lactulose will draw water into the gut until the bowel contents are isotonic with plasma ~300 mOsm/L; bowel contents are eventually excreted which ultimately results in the excretion of water. The suggested target stool output is 1-1.5L/day, which is often achieved by giving between 30-45 ml of lactulose four times daily. The precise change in sodium concentration caused by lactulose is difficult to predict, and should vary case-by-case. For example, in this case report, lactulose dose at 50 mL four times daily increased the serum sodium concentration by 4 mEq/L/day.
Hypertonic saline
Hypertonic saline is usually avoided in patients with hypervolemic hyponatremia with cirrhosis because of the high sodium content which can lead to worsening of edema and ascites. Hypertonic saline is usually reserved for those patients with severe hyponatremia ( Na < 110 mmol/L) or for those who are symptomatic and presenting with seizures, cardiopulmonary distress, or coma. For those with acute, severe, or symptomatic hyponatremia, a 100 ml bolus of 3% saline should be given which can be repeated up to two additional times (a total of 300 mL) if symptoms persist. For those with chronic hyponatremia, 3% saline infusions can be used generally starting at 15-30 mL/hr and titrated to increase the sodium no faster than 6-8 mEq/L/day. As a general guide, the Adrogué-Madias formula calculates an estimated change in sodium based on the composition of 1L of IV fluid administered:
Where ΔNa = expected change in sodium, [Na + K]IVF = the sodium plus potassium concentration of the IV fluid, [Na] is the serum sodium, and TBW = total body water. For example, 3% saline has 513 mmol Na per liter.
Vaptans
Tolvaptan is approved by the FDA for short term treatment of hypervolemic hyponatremia including those with cirrhosis. Tolvaptan works by selectively blocking the action of ADH on vasopressin V2 receptors causing selective water diuresis without affecting sodium or potassium excretion. Meta-analyses have suggested that tolvaptan increased serum sodium and reduced ascites but did not improve overall survival; although it may reduce the risk of hepatic encephalopathy and spontaneous bacterial peritonitis. Tolvaptan use in cirrhosis has been limited due to reports of drug-induced liver injury which include elevated serum aminotransferase and acute liver injury. It is important to note however, that the majority of these reports have been in patients with ADPKD on long term use of tolvaptan (> 30 days).
Overcorrection—osmotic demyelination syndrome
Osmotic demyelination syndrome (ODS) can be a devastating neurologic complication for patients with chronic hyponatremia who experience a rapid correction of serum sodium. Immediately after correcting hyponatremia, affected patients may have improved neurologic functions, but a few days later new neurologic deficits emerge which may be permanent. To decrease the risk of ODS, the rate of sodium correction should not exceed 6-8 mmol/L per 24-hours. Cirrhosis itself is associated with susceptibility for ODS, as are common comorbidities like malnutrition and alcohol use. More on preventing ODS can be found in Skeleton Key Group Case 20. The importance of being mindful of the potential danger when diagnosing and treating cases of hyponatremia cannot be overstated.
E: Summary
As you can see, the management of hyponatremia in patients with cirrhosis can be challenging and there is no one-size-fits-all approach. Our patient presented with hypotonic hypervolemic hyponatremia in the setting of cirrhosis, his sodium level on admission was 114 mmol/L. Here’s how we tackled the case:
Day 1 and 2 – sodium and fluid restriction
We initially started him on daily sodium restriction of 2 g and fluid restriction of 800 mL (for example: 800 mL fluid intake – 800 mL 24-hour urine output – 1 L 24-hour insensible losses = net negative 1 L fluid balance). He also received furosemide 20 mg IV once. However, the sodium initially decreased from 114 to 112 by day 2 and diuretics were held. We then gave one 12.5g infusion of 25% albumin followed by a continuous infusion of normal saline at 75 mL/hr.
Day 3 and 4 – lactulose, midodrine, and octreotide
By day 3, the sodium was unchanged at 112 mEq/L, and we subsequently attempted lactulose 20 mg twice daily. In addition, the patient was started on midodrine 5 mg BID and octreotide 100 mcg TID as his systolic BP remained in the 90’s mm Hg. By the end of day 4, the sodium was trending up from 112 to 115 mEq/L.
Day 5 and 6 – adding back furosemide
Finally, after several days of diuretic holiday, we started a furosemide drip at 5 mg/hr for two days resulting in a further net negative fluid balance. As the patient remained with relative hypotension, we increased midodrine to 15 mg TID, resulting in an increase in systolic BP from the to the 130’s mm Hg. With all of these interventions, the sodium steadily rose to 121 mEq/L by day 6 and 136 mEq/L by day 8.
F: Take Home Messages
- Hyponatremia is the most common electrolyte disturbance among those with advanced cirrhosis and is associated with adverse outcomes
- The pathophysiology in cirrhosis is related to portal hypertension and splanchnic vasodilation, decreased renal blood blow, and ultimately activation of the renin-angiotensin-aldosterone system and non-osmotic stimulation of antidiuretic hormone
- Chronic, asymptomatic hyponatremia in cirrhosis generally does not require treatment
- Therapies for hyponatremia in cirrhosis include fluid restriction, correction of hypokalemia, modifying diuretics, consideration of albumin products, blood pressure/hemodynamic support, and hypertonic saline in urgent cases—other therapies, such as vasopressin antagonists, are used in select cases
Reviewed by: Jefferson L. Triozzi, Joel M, Topf, Raad B. Chowdhury, Chi Chu, Matthew A. Sparks, Margaret Deoliveira