Welcome to the 25th case of the Skeleton Key Group, a team of 50-odd nephrology fellows who work together to build a monthly education package for the Renal Fellow Network. These are actual cases (without patient identifying information) that intrigued the treating physician.
Written by: Narjes Alamri, Raad Chowdhury & Nasim Wiegley
Visual Abstract & Infographics: Jade Teakell & Dilushi Wijayaratne
A.The Stem:
A 65-year-old woman with a history of psoriatic arthritis on methotrexate, tobacco use, and small cell lung cancer (diagnosed 3 weeks ago, not yet started on chemotherapy), is admitted for one week of progressive, generalized, and symmetric weakness with intact sensation. Six months prior to her lung cancer diagnosis, she was diagnosed with SIADH of undetermined etiology. She was managed with fluid restriction and salt tablets, her sodium had remained stable as of the last check about 2 months prior to this hospitalization.
Review of Systems:
No loss of appetite (still able to eat 3 small meals per day); no recent infections; no fevers or chills; no nausea, vomiting, or diarrhea.
Vitals on arrival to the ED:
BP: 135/74 mm Hg | Pulse: 68 BPM | Resp: 18 per minute | SpO2: 100% | Weight: 80.7 kg
On exam, she appeared somnolent, but in no acute distress. Neurologic exam showed intact cranial nerves 2-12, 4/5 motor strength in all four extremities, and no sensory loss.
B. The Labs:
At the time of presentation to the hospital:
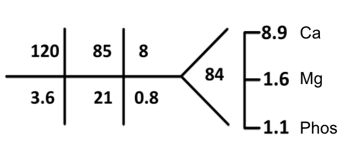
Albumin 3.7 iCa 1.17
The serum sodium of 120 was noted and her previous workup was consistent with SIADH in the setting of small cell lung cancer. Since hyponatremia is not the focus of this particular case, we will proceed to other lab abnormalities noted. See SKG Case 20 for an in-depth look at hyponatremia. Instead let’s focus on the significantly low phosphorus level (1.1 mg/dl) (normal value 2.7-4.5 mg/dl)
C. Working up the Case
Let’s Review Symptoms of Hypophosphatemia:
Effects of hypophosphatemia may involve multiple organ systems, as shown in the figure below. Our patient only had generalized weakness, although this was probably multifactorial.
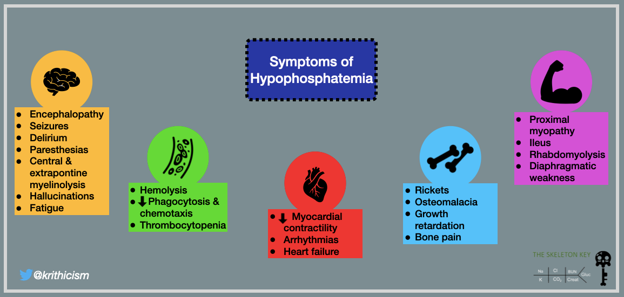
Fig 1. Symptoms of Hypophosphatemia. From The Skeleton Key Group: Case 9
Differential Diagnosis for Hypophosphatemia:
Before we dive into that, let’s first describe the differences between Phosphate, Phosphorous, and Phosphorus. These are often used interchangeably, but important distinctions should be noted. PhosphorOUS is used with something containing phosphorus and is an adjective of phosphorus. PhosphorUS is a chemical element with the symbol of P and atomic number 15. Because phosphorus is highly reactive, it is never found as a free element on earth. Elemental phosphorus exists as either white phosphorus and red phosphorus. In life, phosphorus is found in the form of phosphates (in all life forms). Phosphates occur in both organic and inorganic forms. Organic forms include phospholipids (hence the name phospho). Inorganic forms exist either as free inorganic phosphate ions (PO43−), bound to protein, or complexed with calcium, magnesium, or sodium. Dark colored soft drinks (with the exception of root beer) contain phosphorus in the form of phosphoric acid. This additive gives them their taste of tartness. Sodium phosphate is often used as a food additive to processed food as a thickening agent and preservative.
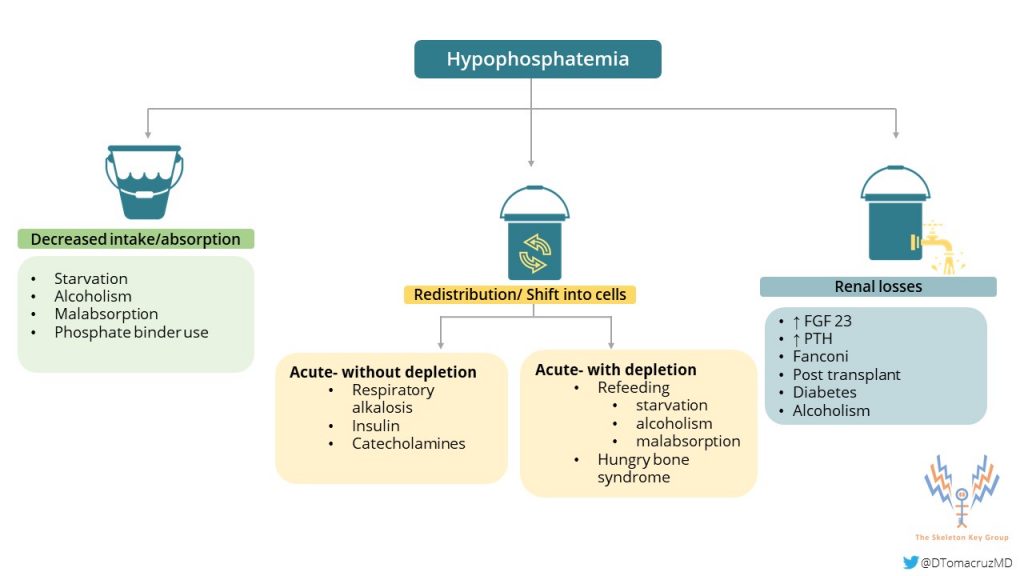
Hypophosphatemia (the inorganic version) can occur by three main mechanisms:
- Redistribution of phosphate can occur in cases of increased insulin secretion after a carbohydrate load, such as refeeding syndrome. Additionally, It can happen during the treatment of diabetic ketoacidosis or alcoholic ketoacidosis. Other conditions that are associated with this mechanism include acute respiratory alkalosis or hungry bone syndrome following parathyroidectomy.
- Decreased intestinal absorption in cases such as malabsorption disorders and use of medications such as phosphate binders and aluminum-based antacids, also decrease phosphorus levels.
- Kidney losses of phosphate can be seen in vitamin D resistance or deficiency, hypophosphatemic rickets, inherited urinary phosphate wasting syndromes, or any disorder leading to proximal tubular dysfunction (Fanconi syndrome). See Case 9 for another unique cause of renal phosphorus wasting. In patients receiving dialysis, especially continuous modalities, phosphate losses from dialysis are an important iatrogenic cause of hypophosphatemia.
Pathophysiology
We will start by reviewing phosphate homeostasis which is regulated by three competing factors:
- Intestinal uptake of phosphate
- Bone absorption and release of phosphate
- Kidney phosphate excretion
Phosphate is absorbed through the entire colon via two pathways: sodium dependent active transport and nondependent passive diffusion. After phosphate is absorbed, the majority ends up stored in bone. A small amount circulates in the extracellular fluid which is eventually filtered by the glomerulus. Filtered phosphate is then reabsorbed in the proximal tubule. The main regulators of phosphate homeostasis are parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and 1,25(OH)2-vitamin D, as you may recall from Case 9.
D. More Data
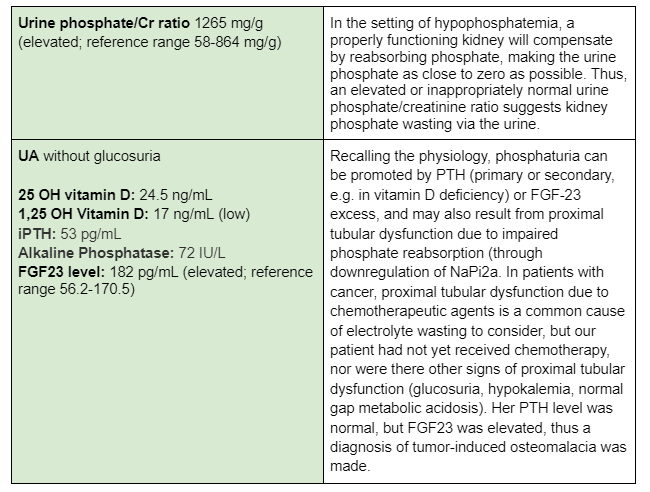
In this particular case, there was little clinical evidence to suggest that there was decreased intestinal absorption or intake and minimal concern for phosphate redistribution. The first step in assessing renal handling of phosphate would be to calculate a spot urine phosphate/creatinine ratio. Alternatively, one can do a 24-hour urine collection or calculate the tubular reabsorption of phosphate or fractional excretion of phosphate.
Further evaluation showed:
E. Final Diagnosis
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome of isolated kidney phosphate wasting caused by ectopic expression of FGF23. The main tumors associated with tumor-induced osteomalacia are mesenchymal tumors; examples include osteosarcoma, rhabdomyosarcoma, and mesothelioma. FGF23 decreases phosphate reabsorption and production of 1,25(OH)2-vitamin D by the kidney, via FGF receptor 1 signaling. FGF23 also decreases phosphate transporters and increases net excretion of phosphate by the kidney. The resulting hypophosphatemia causes rickets, osteomalacia, bone pain, muscle weakness, and fractures. Serum levels of FGF23 usually decrease to normal following resection/treatment of the responsible tumors. These findings are consistent with a pathogenic role for FGF23 in most patients with tumor-induced osteomalacia.
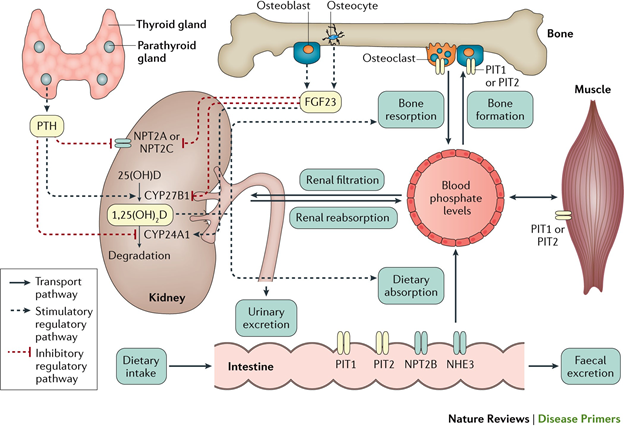
We found one reported case of small cell carcinoma with evidence of elevated plasma FGF23 as well as strong immunostaining for FGF23 in the tumor. Other malignancies that have been implicated include prostate cancer, multiple myeloma, and ovarian cancer.
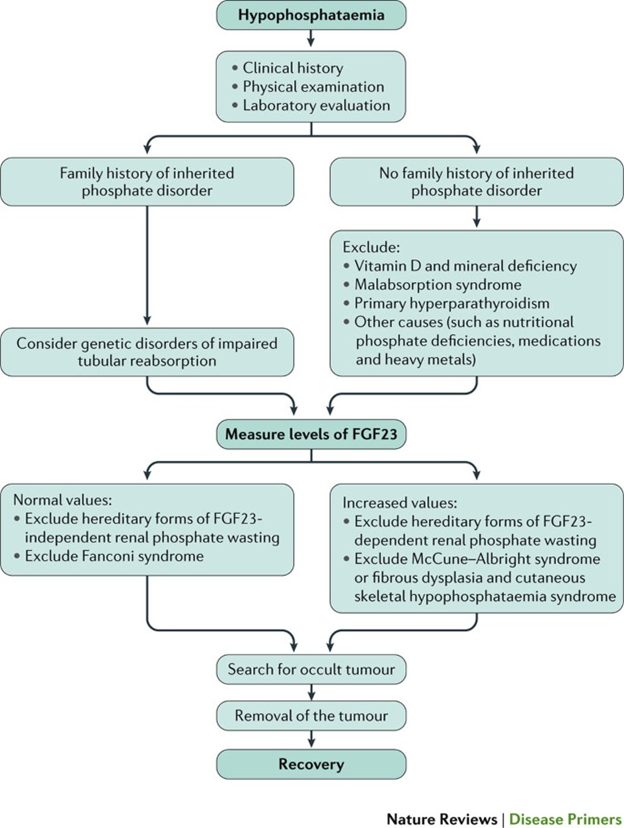
The next graph gives an overall algorithm help to diagnose tumor-induced osteomalacia.
Management
Treatment of TIO is treating the underlying malignancy, plus phosphate repletion, and vitamin D analog
Our patient was started on paclitaxel/carboplatin for her small cell lung cancer. Meanwhile she was prescribed phosphate supplements and calcitriol, and after her second cycle, her weakness and phosphate level started to improve, and phosphate supplementation was no longer required to maintain normal phosphate levels.
F. Take home message:
- Identifying symptoms of hypophosphatemia help early detection and avoid serious complications of hypophosphatemia
- There are three mechanisms for hypophosphatemia; transcellular shift, GI losses, and increased renal excretion.
- FGF23 increases kidney phosphorus wasting.
- Keep TIO in mind when you see a patient with hypophosphatemia and malignancy

Edited by Matthew Sparks



Great review! Thanks!
Very interesting case.